What Is The Chemical Equation For Aerobic Respiration
Juapaving
May 13, 2025 · 5 min read
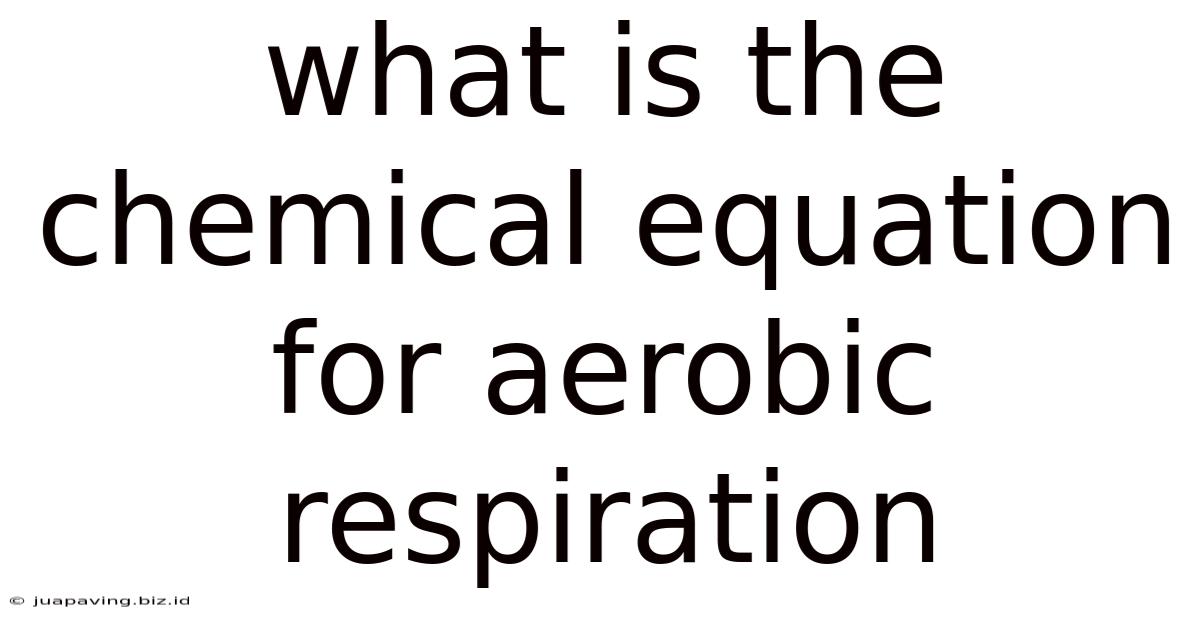
Table of Contents
What is the Chemical Equation for Aerobic Respiration? A Deep Dive into Cellular Energy Production
Aerobic respiration, the process by which cells break down glucose in the presence of oxygen to produce energy, is fundamental to life as we know it. Understanding its chemical equation is key to grasping the intricacies of cellular metabolism and energy transfer. While a simplified equation often suffices for introductory purposes, a more nuanced understanding requires delving into the complex biochemical pathways involved. This article will explore the chemical equation for aerobic respiration, examining both the simplified and the more detailed representations, along with a discussion of the critical steps and molecules involved.
The Simplified Equation: A General Overview
The most common and simplified representation of aerobic respiration is:
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + Energy (ATP)
This equation tells us that one molecule of glucose (C₆H₁₂O₆) reacts with six molecules of oxygen (6O₂) to produce six molecules of carbon dioxide (6CO₂), six molecules of water (6H₂O), and a significant amount of energy stored in the form of adenosine triphosphate (ATP). This equation, while accurate in its overall stoichiometry, significantly simplifies the extremely complex series of reactions that actually constitute aerobic respiration.
Understanding the Reactants and Products
-
Glucose (C₆H₁₂O₆): The primary fuel source for aerobic respiration. This simple sugar is broken down to release energy stored within its chemical bonds. Glucose is obtained through the digestion of carbohydrates in our diet.
-
Oxygen (6O₂): The final electron acceptor in the electron transport chain, the crucial stage of aerobic respiration where the majority of ATP is produced. Oxygen's high electronegativity drives the process, creating a strong electrochemical gradient.
-
Carbon Dioxide (6CO₂): A waste product of aerobic respiration, exhaled from the lungs in animals and released into the atmosphere by plants.
-
Water (6H₂O): Another waste product, formed during the final steps of electron transport.
-
Energy (ATP): The primary energy currency of the cell. ATP is a nucleotide that stores energy in its high-energy phosphate bonds. This energy is used to power a vast array of cellular processes, including muscle contraction, protein synthesis, and active transport.
Delving Deeper: The Stages of Aerobic Respiration
The simplified equation masks the true complexity of aerobic respiration, which occurs in three main stages:
-
Glycolysis: This anaerobic process takes place in the cytoplasm and breaks down glucose into two molecules of pyruvate (C₃H₄O₃). A small amount of ATP is generated directly during glycolysis.
-
Krebs Cycle (Citric Acid Cycle): Occurring in the mitochondria, the Krebs cycle further oxidizes pyruvate, producing carbon dioxide, ATP, NADH, and FADH2. NADH and FADH2 are electron carriers that transport high-energy electrons to the next stage.
-
Electron Transport Chain (ETC) and Oxidative Phosphorylation: Located in the inner mitochondrial membrane, the ETC uses the electrons from NADH and FADH2 to create a proton gradient across the membrane. This gradient drives ATP synthesis through a process called chemiosmosis. Oxygen acts as the final electron acceptor, forming water.
A More Detailed Look at the Chemical Equations:
While a single equation cannot fully encapsulate the entirety of aerobic respiration, we can break it down into equations representing the key stages:
1. Glycolysis:
The net equation for glycolysis is:
Glucose (C₆H₁₂O₆) + 2NAD⁺ + 2ADP + 2Pi → 2Pyruvate (C₃H₄O₃) + 2NADH + 2ATP + 2H₂O
This shows the conversion of glucose into two pyruvate molecules, with a net production of 2 ATP molecules. Note that NAD⁺ is reduced to NADH, carrying electrons to the later stages.
2. Krebs Cycle:
The Krebs cycle involves multiple steps, but the overall equation summarizing its contribution is:
2 Pyruvate (C₃H₄O₃) + 8NAD⁺ + 2FAD + 2ADP + 2Pi + 6H₂O → 6CO₂ + 8NADH + 2FADH₂ + 2ATP + 8H⁺
This equation demonstrates the complete oxidation of pyruvate, generating carbon dioxide and significantly more NADH and FADH2, essential for the electron transport chain.
3. Electron Transport Chain and Oxidative Phosphorylation:
The ETC doesn't have a single, straightforward equation. However, we can represent the overall process as:
10NADH + 2FADH₂ + O₂ → 10NAD⁺ + 2FAD + H₂O + ~34ATP
This is a highly simplified representation. The actual number of ATP molecules produced varies depending on the efficiency of the electron transport chain and the specific organism. The critical aspect is that the electrons from NADH and FADH2 are used to pump protons across the mitochondrial membrane, creating the proton gradient that drives ATP synthase to produce ATP.
Putting it All Together: A Comprehensive Perspective
Combining the simplified equations from each stage does not provide a completely accurate depiction of the entire process due to the intricacies of intermediate metabolites and the variable ATP yield. Nonetheless, it gives a reasonable approximation of the overall stoichiometry:
Combining the simplified equations, we get a more complex but still relatively simplified version:
C₆H₁₂O₆ + 6O₂ + 38ADP + 38Pi → 6CO₂ + 6H₂O + 38ATP
This equation accounts for the approximate net ATP production. However, it's crucial to understand that this number can vary slightly depending on the efficiency of the mitochondrial machinery and the specific conditions.
Beyond the Equations: The Importance of Understanding the Process
While the chemical equations provide a framework for understanding aerobic respiration, they are only a starting point. The true power of understanding this process lies in comprehending the intricate biochemical mechanisms, enzyme regulation, and the interconnectivity of metabolic pathways.
Factors such as:
-
Enzyme activity and regulation: The rate of each reaction in aerobic respiration is carefully controlled by enzymes, which are sensitive to various factors like temperature, pH, and the availability of substrates and cofactors.
-
Redox reactions: The transfer of electrons during the ETC is a series of redox reactions, involving oxidation (loss of electrons) and reduction (gain of electrons).
-
Chemiosmosis: The generation of ATP through chemiosmosis is a remarkable example of energy conversion, harnessing the energy stored in the proton gradient to drive ATP synthesis.
-
Metabolic regulation: The entire process is highly regulated to meet the energy demands of the cell.
Conclusion:
The chemical equation for aerobic respiration, while readily represented in a simplified form, hides a vastly complex network of biochemical reactions. Understanding both the simplified equation and the intricacies of the individual stages — glycolysis, the Krebs cycle, and the electron transport chain — is crucial for a comprehensive grasp of cellular energy production. This knowledge is fundamental to various fields, including medicine, biology, and biotechnology. A deep understanding beyond the equations is necessary for a holistic view of this essential process. Further exploration into the individual steps and regulatory mechanisms unveils the remarkable efficiency and precision of aerobic respiration.
Latest Posts
Latest Posts
-
Greatest Common Factor 15 And 20
May 13, 2025
-
What Is The Gcf Of 12
May 13, 2025
-
Which Gland Has Both Endocrine And Exocrine Functions
May 13, 2025
-
Which Of These Is A Density Independent Factor
May 13, 2025
-
What Is The Molecular Mass Of Na2o
May 13, 2025
Related Post
Thank you for visiting our website which covers about What Is The Chemical Equation For Aerobic Respiration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.