What Is The Basic Unit Of Protein
Juapaving
Mar 12, 2025 · 7 min read
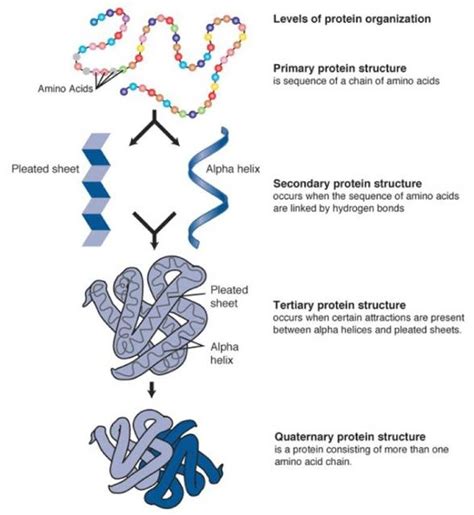
Table of Contents
What is the Basic Unit of Protein?
Proteins are the workhorses of the cell, carrying out a vast array of functions essential for life. From catalyzing biochemical reactions to providing structural support, proteins' versatility stems from their incredibly diverse structures. But to understand how proteins achieve this structural and functional diversity, we must first delve into their fundamental building blocks: amino acids.
Amino Acids: The Building Blocks of Proteins
The basic unit of a protein is the amino acid. These organic molecules are characterized by a central carbon atom (the alpha carbon) bonded to four different chemical groups:
- An amino group (-NH₂): This is a basic group, meaning it can accept a proton (H⁺).
- A carboxyl group (-COOH): This is an acidic group, meaning it can donate a proton (H⁺).
- A hydrogen atom (-H): A simple hydrogen atom.
- A side chain (R-group): This is the variable group, and it's what distinguishes one amino acid from another. The R-group can be anything from a simple hydrogen atom (as in glycine) to a complex aromatic ring structure (as in tryptophan).
It's this R-group variability that accounts for the vast diversity of proteins found in nature. The properties of the R-group, such as its size, charge, polarity, and hydrophobicity, determine the overall properties and function of the protein it contributes to.
The Twenty Standard Amino Acids
There are twenty standard amino acids commonly found in proteins. These amino acids are encoded by the genetic code and are used by ribosomes to synthesize proteins. These twenty amino acids can be categorized based on their R-group properties:
-
Nonpolar, aliphatic amino acids: These amino acids have hydrophobic (water-fearing) side chains. Examples include glycine, alanine, valine, leucine, isoleucine, and methionine. These amino acids tend to cluster together in the interior of proteins, away from the aqueous environment.
-
Aromatic amino acids: These amino acids possess aromatic rings in their side chains. Examples include phenylalanine, tyrosine, and tryptophan. These amino acids are often involved in interactions with other molecules and can absorb ultraviolet light.
-
Polar, uncharged amino acids: These amino acids have hydrophilic (water-loving) side chains that can form hydrogen bonds. Examples include serine, threonine, cysteine, asparagine, and glutamine. These amino acids are often found on the surface of proteins, interacting with the surrounding water molecules.
-
Positively charged (basic) amino acids: These amino acids have positively charged side chains at physiological pH. Examples include lysine, arginine, and histidine. These amino acids often participate in ionic interactions with negatively charged molecules.
-
Negatively charged (acidic) amino acids: These amino acids have negatively charged side chains at physiological pH. Examples include aspartic acid and glutamic acid. These amino acids also participate in ionic interactions with positively charged molecules.
Peptide Bonds: Linking Amino Acids
Amino acids are linked together by peptide bonds to form polypeptide chains. A peptide bond is formed through a dehydration reaction, where a water molecule is removed between the carboxyl group of one amino acid and the amino group of another. This process creates a covalent bond between the two amino acids.
The sequence of amino acids in a polypeptide chain is known as its primary structure. This primary structure dictates the higher-order structures of the protein, influencing its overall three-dimensional shape and, ultimately, its function.
Protein Structure: From Primary to Quaternary
The structure of a protein is crucial to its function. Proteins exhibit four levels of structural organization:
1. Primary Structure
As mentioned earlier, the primary structure is the linear sequence of amino acids in a polypeptide chain. This sequence is determined by the genetic code and is crucial for determining the higher-order structures. Even a single amino acid substitution can drastically alter the protein's function, as seen in sickle cell anemia, where a single amino acid change in hemoglobin leads to a misfolded protein and severe health consequences.
2. Secondary Structure
The secondary structure refers to local folding patterns within the polypeptide chain. Two common secondary structures are:
-
Alpha-helices: These are coiled structures stabilized by hydrogen bonds between the carbonyl oxygen of one amino acid and the amide hydrogen of another amino acid four residues down the chain.
-
Beta-sheets: These are sheet-like structures formed by hydrogen bonds between adjacent polypeptide strands. The strands can be parallel or antiparallel, depending on the orientation of the amino acid chains.
Other secondary structures, such as loops and turns, also exist and contribute to the overall protein structure.
3. Tertiary Structure
The tertiary structure refers to the overall three-dimensional arrangement of a polypeptide chain. This structure is stabilized by various interactions between amino acid side chains, including:
-
Hydrophobic interactions: Nonpolar side chains cluster together in the protein's interior, away from water.
-
Hydrogen bonds: Polar side chains form hydrogen bonds with each other or with water molecules.
-
Ionic bonds (salt bridges): Positively and negatively charged side chains attract each other.
-
Disulfide bonds: Covalent bonds formed between cysteine residues.
The tertiary structure is crucial for the protein's function, as it creates the active site (in enzymes) or the binding site (in receptors) necessary for interactions with other molecules.
4. Quaternary Structure
Some proteins consist of multiple polypeptide chains, called subunits, arranged together to form a functional protein complex. This arrangement is known as the quaternary structure. The subunits are held together by the same types of interactions that stabilize tertiary structure. Hemoglobin, for instance, consists of four subunits that work together to transport oxygen in the blood.
Protein Function: A Diverse Array of Roles
The diverse structures of proteins lead to an equally diverse range of functions. Proteins play critical roles in virtually every aspect of cellular life, including:
-
Enzymes: These proteins catalyze biochemical reactions, accelerating the rates of metabolic processes.
-
Structural proteins: These proteins provide structural support to cells and tissues, such as collagen in connective tissue and keratin in hair and nails.
-
Transport proteins: These proteins carry molecules across cell membranes or throughout the body, such as hemoglobin transporting oxygen in the blood.
-
Motor proteins: These proteins generate movement within cells, such as myosin in muscle cells.
-
Hormones: These proteins act as chemical messengers, coordinating various physiological processes.
-
Receptors: These proteins receive signals from the environment, initiating cellular responses.
-
Antibodies: These proteins are part of the immune system, defending against foreign invaders.
Protein Synthesis: From DNA to Protein
The information for protein synthesis is encoded in DNA. The DNA sequence is transcribed into messenger RNA (mRNA), which is then translated into a polypeptide chain by ribosomes. The process of protein synthesis involves several key steps:
-
Transcription: The DNA sequence is copied into an mRNA molecule.
-
mRNA processing: The mRNA molecule undergoes modifications, such as splicing and capping, before it leaves the nucleus.
-
Translation: The mRNA molecule is translated into a polypeptide chain by ribosomes. Transfer RNA (tRNA) molecules carry amino acids to the ribosome, where they are added to the growing polypeptide chain according to the mRNA sequence.
-
Protein folding: The newly synthesized polypeptide chain folds into its three-dimensional structure, assisted by chaperone proteins.
Conclusion: The Importance of Amino Acids and Protein Structure
In summary, the basic unit of a protein is the amino acid. The sequence of amino acids, the primary structure, determines the higher-order structures (secondary, tertiary, and quaternary) of a protein. These structures are crucial for protein function, which is incredibly diverse and essential for life. Understanding the relationship between amino acid sequence, protein structure, and function is key to understanding the complexity and beauty of biological systems. Further research into protein structure and function continues to yield insights into disease mechanisms, drug development, and biotechnology applications. The twenty standard amino acids, arranged in countless combinations, form the foundation of life itself, underscoring the fundamental importance of these building blocks in the biological world. The intricate interplay of forces that shape protein structure – hydrophobic interactions, hydrogen bonds, disulfide bridges, and electrostatic attractions – remains a captivating area of ongoing scientific inquiry, pushing the boundaries of our understanding of biological systems.
Latest Posts
Latest Posts
-
What Is The Difference Between A Purine And A Pyrimidine
May 09, 2025
-
What Are The Basic Units Of Living Matter
May 09, 2025
-
Give The Iupac Names For The Following Compounds
May 09, 2025
-
How Big Is 6 Inches In Cm
May 09, 2025
-
How Many Feet In 85 Inches
May 09, 2025
Related Post
Thank you for visiting our website which covers about What Is The Basic Unit Of Protein . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.