What Is Produced When An Acid Reacts With A Base
Juapaving
Mar 22, 2025 · 6 min read
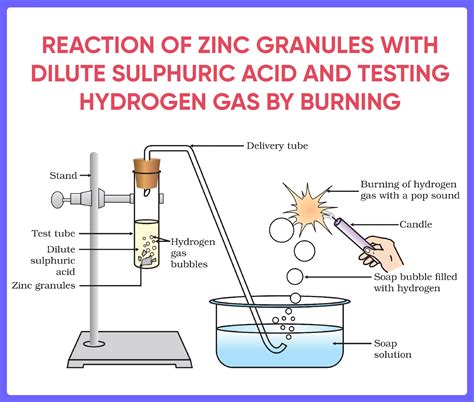
Table of Contents
What is Produced When an Acid Reacts with a Base?
The reaction between an acid and a base is a fundamental concept in chemistry, known as neutralization. Understanding what's produced during this reaction is crucial for comprehending numerous chemical processes, from everyday occurrences like digestion to industrial applications like wastewater treatment. This in-depth article will explore the products of acid-base reactions, the different types of reactions, factors influencing the reaction, and practical applications.
The Core Reaction: Salt and Water
The most common outcome of an acid-base reaction is the formation of salt and water. This is particularly true for reactions involving strong acids and strong bases. Let's break this down:
Acids: Proton Donors
Acids are substances that donate protons (H⁺ ions) when dissolved in water. Examples include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and nitric acid (HNO₃). The strength of an acid depends on its ability to readily donate these protons. Strong acids, like those listed above, completely dissociate in water, while weak acids only partially dissociate.
Bases: Proton Acceptors
Bases are substances that accept protons (H⁺ ions). Common examples are sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)₂). Similar to acids, the strength of a base depends on its ability to accept protons. Strong bases completely dissociate in water, releasing hydroxide ions (OH⁻), while weak bases only partially dissociate.
The Neutralization Process
When an acid and a base react, the protons (H⁺) from the acid combine with the hydroxide ions (OH⁻) from the base to form water (H₂O). This is the essence of neutralization:
H⁺(aq) + OH⁻(aq) → H₂O(l)
The remaining ions, typically a cation from the base and an anion from the acid, combine to form a salt. For instance, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl – table salt) and water:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
This equation illustrates the general principle: acid + base → salt + water. The specific salt formed depends entirely on the acid and base used in the reaction.
Beyond Salt and Water: Exploring Other Products
While salt and water are the primary products of many acid-base reactions, other products can form depending on the reactants and reaction conditions. These include:
Gas Formation:
Certain acid-base reactions produce gases as byproducts. This often occurs when the salt formed is unstable and decomposes, releasing a gas. A classic example is the reaction between a carbonate or bicarbonate and an acid:
CaCO₃(s) + 2HCl(aq) → CaCl₂(aq) + H₂O(l) + CO₂(g)
In this reaction between calcium carbonate (limestone) and hydrochloric acid, carbon dioxide gas is released. Similar reactions occur with other carbonates and bicarbonates, producing various gases depending on the anion present in the salt.
Precipitation Reactions:
If the salt formed during the neutralization reaction is insoluble in water, it precipitates out of the solution as a solid. This is a precipitation reaction, and the solid precipitate is an additional product besides water. For example, the reaction between silver nitrate (AgNO₃) and sodium chloride (NaCl) produces a white precipitate of silver chloride (AgCl):
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
These precipitation reactions are valuable in analytical chemistry for identifying and quantifying ions in solution.
Factors Influencing Acid-Base Reactions
Several factors influence the outcome and efficiency of acid-base reactions:
Strength of the Acid and Base:
Strong acids and bases react more readily and completely than weak acids and bases. Reactions involving weak acids or bases may reach equilibrium before complete neutralization, resulting in a buffer solution – a solution that resists changes in pH.
Concentration of Reactants:
The concentration of both the acid and the base affects the reaction rate and the amount of products formed. Higher concentrations generally lead to faster reactions and greater product yields.
Temperature:
Temperature influences the reaction rate. Higher temperatures typically increase the kinetic energy of the reacting molecules, leading to faster reaction rates.
Presence of Catalysts:
Catalysts can accelerate acid-base reactions without being consumed in the process. However, catalysts are less common in simple neutralization reactions.
Applications of Acid-Base Reactions
Acid-base reactions have widespread applications in various fields:
Industrial Processes:
- Wastewater treatment: Neutralization is used to adjust the pH of wastewater before discharge, protecting the environment. Acids and bases are added to neutralize excess acidity or alkalinity.
- Chemical synthesis: Acid-base reactions are crucial steps in synthesizing many chemicals, pharmaceuticals, and materials.
- Food processing: Acids and bases are used to control the pH of food products, improving taste, preservation, and texture.
Everyday Life:
- Digestion: The stomach produces hydrochloric acid to aid digestion, while the small intestine uses bicarbonate to neutralize excess acid.
- Antacid tablets: These tablets contain bases that neutralize excess stomach acid, relieving heartburn and indigestion.
- Cleaning products: Many cleaning products utilize acids or bases to remove stains, grease, and other substances.
Understanding the pH Scale
The pH scale is a logarithmic scale that measures the acidity or alkalinity of a solution. A pH of 7 is neutral, while values below 7 indicate acidity and values above 7 indicate alkalinity. The pH of the resulting solution after an acid-base reaction depends on the strength and concentration of the reacting acid and base. A complete neutralization of a strong acid with a strong base results in a solution with a pH of 7. However, neutralization involving weak acids or bases might result in solutions with pH values different from 7.
Titration: A Quantitative Approach
Titration is a laboratory technique used to determine the concentration of an unknown acid or base by reacting it with a solution of known concentration. This process involves carefully adding a solution of known concentration (the titrant) to a solution of unknown concentration until the reaction is complete. The point at which the reaction is complete is called the equivalence point, and it is often indicated by a color change using an indicator. Titration provides a quantitative measure of the acid or base concentration, offering valuable insights into the stoichiometry of acid-base reactions.
Conclusion: The Versatility of Acid-Base Reactions
The reaction between an acid and a base is a fundamental chemical process with far-reaching consequences. While the formation of salt and water is a common outcome, the products can vary depending on the reactants and conditions. Understanding these reactions is essential for comprehending various chemical processes, from everyday occurrences to complex industrial applications. The versatility of acid-base reactions, coupled with techniques like titration, makes them invaluable tools in various scientific fields and industrial processes. The detailed exploration of this reaction demonstrates the intricate relationship between acids, bases, and the resulting products, highlighting its significance in chemistry and beyond. From industrial applications to everyday life, acid-base reactions play a pivotal role in shaping our world. Further research into specific reactions and their applications will continue to unveil the vast potential of this fundamental chemical phenomenon.
Latest Posts
Latest Posts
-
What Is The Lcm Of 3
Mar 23, 2025
-
What Is The Amount Of Matter In An Object Called
Mar 23, 2025
-
How Many Subsets In A Set
Mar 23, 2025
-
Velocity Is The Rate Of Change Of
Mar 23, 2025
-
How Does The Digestive System Help The Body Maintain Homeostasis
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is Produced When An Acid Reacts With A Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
