What Is Lone Pair And Bond Pair In Chemistry
Juapaving
Mar 24, 2025 · 7 min read
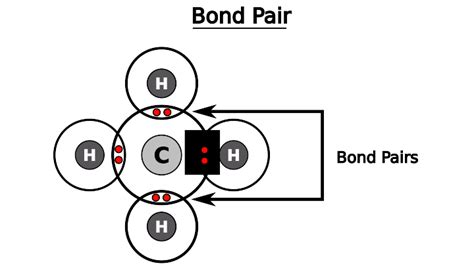
Table of Contents
What is a Lone Pair and Bond Pair in Chemistry? A Comprehensive Guide
Understanding the concepts of lone pairs and bond pairs is fundamental to grasping the structure and behavior of molecules in chemistry. These concepts are crucial for predicting molecular geometry, polarity, and reactivity. This comprehensive guide will delve deep into the definitions, differences, and implications of lone pairs and bond pairs, illustrated with numerous examples.
Defining Lone Pairs and Bond Pairs
At the heart of understanding molecular structure lies the concept of valence electrons – the outermost electrons in an atom that participate in chemical bonding. These electrons can be categorized into two types:
Lone Pairs (Non-bonding Pairs)
A lone pair, also known as a non-bonding pair or unshared pair, refers to a pair of valence electrons that are not involved in covalent bonding. These electrons are associated solely with one atom and are localized around it. Think of them as electrons that are "unpaired" and not participating in the sharing process that defines a covalent bond. They significantly influence the molecule's shape and properties.
Bond Pairs (Shared Pairs)
A bond pair, also known as a shared pair or bonding pair, is a pair of valence electrons that are shared between two atoms to form a covalent bond. This sharing creates a stable electron configuration for both atoms involved, fulfilling the octet rule (or duet rule for hydrogen) and resulting in a strong attractive force that holds the atoms together.
The Octet Rule and its Implications
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a full outer electron shell of eight electrons. This stable configuration is similar to that of a noble gas. However, there are exceptions to the octet rule. Understanding the octet rule is crucial because it helps predict how many electrons will be involved in bonding and, consequently, how many lone pairs and bond pairs an atom might possess.
For example, consider the oxygen atom (O). Oxygen has six valence electrons. To achieve an octet, it needs two more electrons. It can achieve this by either gaining two electrons to form an oxide ion (O²⁻) or by sharing two electrons through covalent bonds. In a water molecule (H₂O), oxygen shares two electrons with each hydrogen atom, forming two bond pairs. The remaining four valence electrons on the oxygen atom form two lone pairs.
Visualizing Lone Pairs and Bond Pairs: Lewis Structures
Lewis structures, also known as Lewis dot diagrams, provide a visual representation of lone pairs and bond pairs in a molecule. They show the valence electrons as dots, with shared pairs represented by lines connecting the atoms.
Example: Water (H₂O)
The Lewis structure for water shows:
- Oxygen (O) with two lone pairs (four dots) and two bond pairs (two lines connecting to the two hydrogen atoms).
- Each Hydrogen (H) atom with one bond pair (one line connecting to the oxygen atom).
Example: Ammonia (NH₃)
Ammonia (NH₃) has:
- Nitrogen (N) with one lone pair (two dots) and three bond pairs (three lines connecting to the three hydrogen atoms).
- Each Hydrogen (H) atom with one bond pair (one line connecting to the nitrogen atom).
Example: Methane (CH₄)
Methane (CH₄) has:
- Carbon (C) with four bond pairs (four lines connecting to four hydrogen atoms) and zero lone pairs.
- Each Hydrogen (H) atom with one bond pair (one line connecting to the carbon atom).
These examples demonstrate how Lewis structures effectively illustrate the distribution of lone pairs and bond pairs within a molecule.
The Influence of Lone Pairs and Bond Pairs on Molecular Geometry
The arrangement of atoms in a molecule, its geometry, is significantly influenced by the presence and distribution of lone pairs and bond pairs. The Valence Shell Electron Pair Repulsion (VSEPR) theory is a powerful tool for predicting molecular geometry based on this principle. VSEPR theory postulates that electron pairs (both bonding and non-bonding) repel each other and try to get as far apart as possible to minimize repulsion.
Lone pairs exert a stronger repulsive force than bond pairs because they are closer to the central atom and occupy more space. Consequently, the presence of lone pairs can significantly distort the molecular geometry from the ideal geometry predicted solely based on the number of bonding pairs.
Examples:
- Methane (CH₄): Four bond pairs and no lone pairs result in a tetrahedral geometry.
- Ammonia (NH₃): Three bond pairs and one lone pair result in a trigonal pyramidal geometry (slightly distorted from tetrahedral).
- Water (H₂O): Two bond pairs and two lone pairs result in a bent or V-shaped geometry (significantly distorted from tetrahedral).
The Impact on Molecular Polarity
The distribution of electrons in a molecule affects its polarity. A polar molecule possesses a net dipole moment, meaning there is a separation of positive and negative charge. Lone pairs contribute significantly to molecular polarity.
Molecules with symmetrical distribution of bond pairs and lone pairs often have nonpolar character (e.g., methane). However, molecules with asymmetrical distribution of electron density, often due to the presence of lone pairs, are typically polar (e.g., water, ammonia). The presence of lone pairs creates regions of higher electron density, which create a negative pole in the molecule, making it polar.
Lone Pairs and Bond Pairs in Different Types of Bonds
The concepts of lone pairs and bond pairs are not limited to simple covalent bonds. They also apply to:
-
Coordinate Covalent Bonds: In coordinate covalent bonds, both electrons in the shared pair originate from the same atom. The atom donating the electron pair has a lone pair before forming the bond, and after the bond formation, the electron pair becomes a bond pair.
-
Multiple Bonds: Multiple bonds (double and triple bonds) involve more than one pair of electrons shared between two atoms. These multiple bonds consist of sigma (σ) bonds and pi (π) bonds. While the sigma bond is similar to a single bond and is involved in determining molecular shape, pi bonds are located above and below the sigma bond and generally don’t significantly influence the VSEPR geometry predictions.
Lone Pairs and Bond Pairs in Advanced Chemistry Concepts
The concepts of lone pairs and bond pairs are not just foundational for understanding basic molecular structures; they extend into more advanced topics:
-
Hybridization: The concept of hybridization explains how atomic orbitals mix to form hybrid orbitals that participate in bonding. The number and type of hybrid orbitals formed are directly related to the number of bond pairs and lone pairs around an atom.
-
Molecular Orbital Theory: This theory provides a more sophisticated description of bonding than VSEPR theory and involves the formation of molecular orbitals from atomic orbitals. The presence of lone pairs affects the energy levels of these molecular orbitals.
-
Reactivity: The presence and location of lone pairs often dictate the reactivity of a molecule. Lone pairs can act as nucleophiles, readily donating electrons to electron-deficient centers. This makes molecules with lone pairs more likely to participate in certain chemical reactions.
Conclusion: The Significance of Lone Pairs and Bond Pairs
Understanding the concepts of lone pairs and bond pairs is crucial for comprehending the structure, properties, and reactivity of molecules. These concepts provide the basis for predicting molecular geometry, polarity, and participation in chemical reactions. From the simple Lewis structures to the advanced concepts of hybridization and molecular orbital theory, the influence of lone pairs and bond pairs is pervasive throughout chemistry. By mastering these concepts, you gain a robust foundation for deeper exploration in the fascinating world of chemical bonding. This understanding allows chemists to predict molecular behavior and design new molecules with specific properties. It is the cornerstone of many aspects of chemistry, including organic chemistry, inorganic chemistry, and biochemistry. Therefore, a thorough grasp of these principles is essential for any aspiring chemist.
Latest Posts
Latest Posts
-
What Direction Does Dna Polymerase Read
Mar 26, 2025
-
What Is 25 Cm In Inches
Mar 26, 2025
-
Which Of The Following Statements Describes The Process Of Globalization
Mar 26, 2025
-
What Are The Greatest Common Factors Of 48
Mar 26, 2025
-
The Unit Of Energy In S I Units Is
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is Lone Pair And Bond Pair In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
