What Is Difference Between Gas And Vapour
Juapaving
Apr 06, 2025 · 5 min read
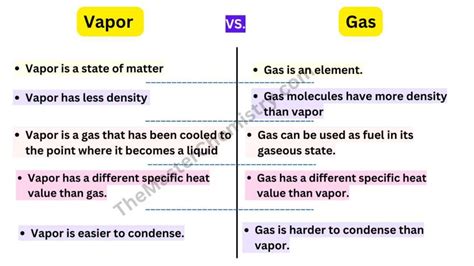
Table of Contents
What's the Difference Between Gas and Vapor? A Deep Dive
The terms "gas" and "vapor" are often used interchangeably in casual conversation, leading to confusion. While they both represent the gaseous phase of matter, there's a crucial distinction based on their relationship to their liquid or solid phase at a given temperature and pressure. Understanding this difference is key to various scientific and engineering applications. This comprehensive guide will explore the nuances between gases and vapors, examining their properties, behaviors, and real-world examples.
Defining Gas and Vapor: A Fundamental Distinction
The core difference lies in the substance's boiling point. A gas exists as a gas at standard temperature and pressure (STP), meaning room temperature and atmospheric pressure. It doesn't readily condense into a liquid or solid under normal conditions. Think of oxygen (O₂), nitrogen (N₂), or hydrogen (H₂). These are naturally gaseous and require significant changes in temperature and/or pressure to transition to a liquid or solid phase.
A vapor, on the other hand, is a gaseous phase of a substance that exists as a liquid or solid at standard temperature and pressure. It is essentially a gas that can readily condense back into its liquid or solid state under normal conditions. Water vapor is a perfect example. Water is a liquid at room temperature, but when heated, it transitions into water vapor—a gas that can readily condense back into liquid water if cooled.
Critical Temperature and Pressure: The Key Differentiators
The behavior of a substance as a gas or vapor is intrinsically linked to its critical temperature and critical pressure.
-
Critical Temperature: This is the temperature above which a substance cannot be liquefied, no matter how much pressure is applied. Above this critical temperature, the substance exists solely as a gas, regardless of pressure.
-
Critical Pressure: This is the pressure required to liquefy a substance at its critical temperature. At temperatures below the critical temperature, increasing the pressure will eventually liquefy the substance. However, above the critical temperature, increasing pressure only increases the density of the gaseous phase; it won't result in liquefaction.
Substances with low critical temperatures are more likely to exist as vapors under normal conditions, while those with high critical temperatures are more likely to be gases.
Exploring the Properties of Gases and Vapors
While both gases and vapors share some similar properties—they occupy the entire available volume, they're compressible, and they exhibit random molecular motion—there are key differences in their behavior:
1. Condensation and Saturation
Vapors readily condense into their liquid or solid phase when the temperature decreases or the pressure increases. This condensation process can lead to saturation, where the air holds the maximum amount of vapor it can at a given temperature and pressure. Exceeding this saturation point results in condensation. Gases, on the other hand, typically require much more extreme changes in temperature and pressure to condense.
2. Density
Vapors generally have lower densities than gases at the same temperature and pressure. This is because the molecules in a vapor are further apart than in a gas, reflecting their proximity to their liquid or solid state.
3. Behavior under Pressure
The response of gases and vapors to pressure changes also differs. Vapors are more sensitive to pressure changes; a slight increase in pressure can cause significant condensation. Gases are less susceptible to condensation from pressure changes alone, often requiring significant pressure increases along with temperature decreases.
Real-World Examples: Illustrating the Difference
Let's look at some real-world examples to solidify our understanding:
-
Water: Water vapor is a vapor because water exists as a liquid at STP. Water vapor in the atmosphere can condense to form clouds, rain, or dew depending on temperature and pressure conditions.
-
Oxygen: Oxygen is a gas because it remains gaseous at STP. Significant cooling and/or compression are required to liquefy oxygen.
-
Freon (Refrigerant): Many refrigerants, like Freon, are vapors. These substances are liquids under normal conditions but readily vaporize when pressure is reduced, enabling cooling through evaporation.
-
Carbon Dioxide: At room temperature and atmospheric pressure, carbon dioxide is a gas. However, under specific high-pressure conditions (like in a carbonated drink), it exists as a supercritical fluid—a state that's neither liquid nor gas but shares characteristics of both.
-
Mercury: Mercury is a liquid at room temperature, but when heated, it forms mercury vapor, a hazardous substance.
Practical Implications: Why Understanding the Distinction Matters
Understanding the difference between gases and vapors has significant implications across various fields:
-
Meteorology: Accurate weather forecasting relies on understanding the behavior of water vapor in the atmosphere, including its role in cloud formation, precipitation, and humidity.
-
Chemical Engineering: Designing and operating chemical processes requires a thorough understanding of vapor-liquid equilibria (VLE) and phase transitions, crucial for efficient separation and purification processes.
-
Refrigeration and Air Conditioning: The principles governing vaporization and condensation are fundamental to refrigeration and air conditioning technologies, utilizing the phase transitions of refrigerants to achieve cooling.
-
Environmental Science: Understanding the behavior of gaseous pollutants and vapors is crucial for air quality monitoring and pollution control strategies.
-
Material Science: The evaporation and condensation of vapors are vital in processes like thin-film deposition and material synthesis.
Conclusion: Gas vs. Vapor—A Critical Distinction
In conclusion, while both gases and vapors represent matter in a gaseous state, the crucial distinction lies in their relationship to their liquid or solid phase at standard temperature and pressure. Gases exist as gases under normal conditions, whereas vapors are gases that can readily condense into a liquid or solid state. This distinction, driven by critical temperature and pressure, has significant implications across various scientific and engineering disciplines. By understanding this fundamental difference, we can gain a deeper appreciation for the complex behavior of matter and its practical applications in various fields. Further research into the specifics of various substances' phase diagrams provides a more profound understanding of gas-vapor interactions. Understanding this nuanced difference unlocks a wealth of knowledge and its real-world applications.
Latest Posts
Latest Posts
-
85 Inches Is How Many Feet
Apr 08, 2025
-
Words That Begin With K Preschool
Apr 08, 2025
-
What Temp Is Celsius And Fahrenheit The Same
Apr 08, 2025
-
Sum Of Interior Angles Of Trapezoid
Apr 08, 2025
-
What Is The Lowest Common Multiple Of 7 And 8
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about What Is Difference Between Gas And Vapour . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.