What Is A Solution And A Mixture
Juapaving
Mar 28, 2025 · 5 min read
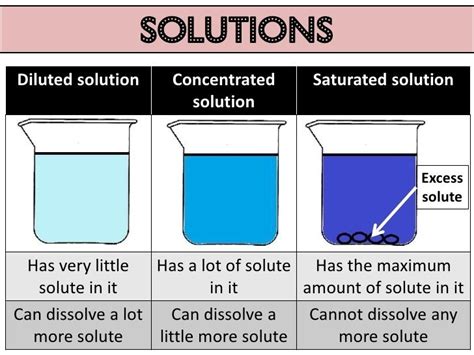
Table of Contents
What is a Solution and a Mixture? A Deep Dive into Chemistry
Understanding the difference between a solution and a mixture is fundamental to grasping basic chemistry concepts. While both involve combining two or more substances, the nature of that combination dictates whether the result is a solution or a mixture. This comprehensive guide will delve into the definitions, properties, types, and examples of solutions and mixtures, helping you to confidently differentiate between them.
Defining Solutions and Mixtures: Key Differences
Before we embark on a detailed exploration, let's establish the core distinction:
Mixture: A mixture is a substance composed of two or more components not chemically bonded. A key characteristic is that the components retain their individual chemical properties. They can be physically separated using methods like filtration, distillation, evaporation, or chromatography.
Solution: A solution is a homogeneous mixture. This means that the components are uniformly distributed throughout the solution at a molecular level. A solution consists of a solute (the substance being dissolved) and a solvent (the substance doing the dissolving). The solute particles are completely dispersed within the solvent, making it impossible to visually distinguish the individual components.
Homogeneous vs. Heterogeneous Mixtures: A Crucial Distinction
The term "homogeneous" is crucial in differentiating solutions from other types of mixtures. Let's clarify this:
-
Homogeneous Mixture: The components are evenly distributed, creating a uniform composition throughout. Solutions are a type of homogeneous mixture. Examples include saltwater, air, and sugar dissolved in water. You cannot visually distinguish the different components.
-
Heterogeneous Mixture: The components are not evenly distributed. Different regions of the mixture will have different compositions. Examples include sand and water, oil and water, and a salad. You can visually distinguish the different components.
Properties of Solutions and Mixtures
Understanding the properties of solutions and mixtures helps us further distinguish them.
Solutions: Unique Characteristics
-
Uniform Composition: As mentioned, the key feature is uniform composition throughout. No matter where you sample the solution, the concentration of solute and solvent will be identical.
-
Particle Size: Solute particles in a solution are extremely small – typically at the atomic or molecular level. This ensures complete dispersion and transparency (unless the solution is inherently colored due to the solute's properties).
-
Filtration: Solutions cannot be separated by simple filtration. The solute particles are too small to be trapped by a filter paper.
-
Solubility: The maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature is its solubility. Solubility is a crucial factor in determining whether a solution can be formed.
Mixtures: Variable Properties
-
Variable Composition: Unlike solutions, mixtures have variable compositions. The ratio of components can fluctuate.
-
Particle Size: Mixture components can have a wide range of particle sizes, from microscopic to macroscopic.
-
Separation: Mixtures can often be separated into their components using physical methods. The specific method depends on the properties of the components.
-
Retain Individual Properties: The components of a mixture retain their individual chemical properties. For instance, in a mixture of sand and iron filings, the sand remains sand, and the iron retains its magnetic properties.
Types of Solutions and Mixtures
Let's explore the various types of solutions and mixtures based on their composition and properties.
Types of Solutions:
-
Solid Solutions: The solute is a solid dissolved in a solid solvent (e.g., alloys like brass – a mixture of copper and zinc).
-
Liquid Solutions: The most common type. The solute is dissolved in a liquid solvent (e.g., saltwater, sugar dissolved in water).
-
Gaseous Solutions: Gases dissolved in gaseous solvents (e.g., air – a mixture of nitrogen, oxygen, and other gases).
Types of Mixtures:
-
Suspensions: Heterogeneous mixtures where the solute particles are relatively large and tend to settle out over time (e.g., muddy water).
-
Colloids: Heterogeneous mixtures with particles larger than those in a solution but smaller than those in a suspension. The particles remain dispersed and don't settle out easily (e.g., milk, fog).
Examples of Solutions and Mixtures in Everyday Life
To solidify your understanding, let's look at some real-world examples:
Examples of Solutions:
- Seawater: Salt (sodium chloride) dissolved in water.
- Air: A mixture of gases where nitrogen and oxygen are the main components, considered a solution because it's homogeneous.
- Sugar in water: A simple and common example of a liquid solution.
- Brass: An alloy, a solid solution of copper and zinc.
Examples of Mixtures:
- Sand and water: A heterogeneous mixture; the sand particles settle out.
- Oil and water: A heterogeneous mixture; the oil and water layers separate.
- Salad: A heterogeneous mixture of various vegetables and dressings.
- Muddy water: A suspension where soil particles are suspended in water.
Factors Affecting Solubility in Solutions
Several factors influence how much solute can dissolve in a solvent:
-
Temperature: Generally, increasing the temperature increases the solubility of solids in liquids. The effect on gases is the opposite; increased temperature reduces solubility.
-
Pressure: Pressure significantly affects the solubility of gases in liquids. Higher pressure increases solubility.
-
Nature of Solute and Solvent: "Like dissolves like" is a crucial principle. Polar solvents dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes.
Applications of Solutions and Mixtures
Solutions and mixtures are ubiquitous in various fields:
-
Medicine: Many medicines are solutions or suspensions administered orally or intravenously.
-
Industry: Many industrial processes rely on solutions and mixtures for chemical reactions, cleaning, and other applications.
-
Agriculture: Fertilizers are often solutions or mixtures of various nutrients.
-
Food Science: Many food products are solutions or mixtures (e.g., soft drinks, sauces).
Advanced Concepts: Concentration of Solutions
The concentration of a solution refers to the amount of solute present in a given amount of solvent or solution. This can be expressed in various ways:
-
Molarity (M): Moles of solute per liter of solution.
-
Molality (m): Moles of solute per kilogram of solvent.
-
Percent by mass (% w/w): Grams of solute per 100 grams of solution.
-
Percent by volume (% v/v): Milliliters of solute per 100 milliliters of solution.
Conclusion: Mastering the Distinctions
Understanding the difference between solutions and mixtures is crucial for anyone studying chemistry or related fields. The distinction hinges on the homogeneity of the mixture and the interaction between the components. Remembering that solutions are homogeneous mixtures where the solute is completely dissolved at a molecular level, while mixtures can be either homogeneous or heterogeneous, provides a solid foundation for further explorations in chemistry. By applying this knowledge, we can better comprehend numerous natural phenomena and technological applications in our daily lives.
Latest Posts
Latest Posts
-
Worksheet On Passive And Active Voice
Mar 31, 2025
-
A Piece Of Land Surrounded By Water On Three Sides
Mar 31, 2025
-
How Far Is 20 Kilometers In Miles
Mar 31, 2025
-
How To Find The Perimeter Of Semicircle
Mar 31, 2025
-
What Is The Least Common Multiple Of 11 And 2
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is A Solution And A Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
