What Is A Horizontal Row Called On The Periodic Table
Juapaving
Mar 05, 2025 · 7 min read
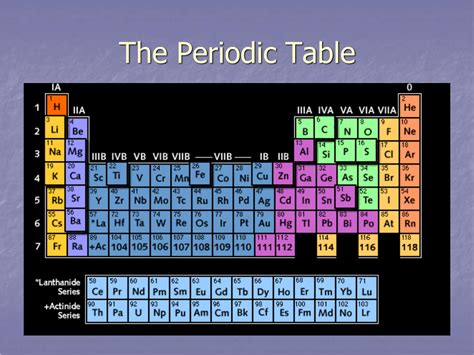
Table of Contents
What is a Horizontal Row Called on the Periodic Table? Understanding Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding its structure is key to grasping the relationships between elements and predicting their behavior. A frequently asked question revolves around the horizontal rows of the periodic table: what is a horizontal row called? The answer is simple: a period. But understanding why these horizontal rows are called periods and what their significance is requires a deeper dive. This comprehensive article will explore periods, their properties, and their crucial role in chemistry.
Defining a Period: More Than Just a Horizontal Row
A period in the periodic table represents a horizontal row of elements. These rows are numbered from 1 to 7, with each number corresponding to the highest principal quantum number (n) of the electrons in that period's elements. The principal quantum number describes the energy level of an electron and dictates the element's position on the table. This seemingly simple arrangement holds immense significance, revealing crucial trends in atomic size, ionization energy, and electronegativity.
The Significance of Principal Quantum Number (n)
The principal quantum number (n) is directly tied to the period number. Elements in Period 1 have electrons only in the first energy level (n=1), elements in Period 2 have electrons in the first and second energy levels (n=1 and n=2), and so on. This fundamental relationship is the basis for the periodic table's organization and the underlying reason why periods exhibit predictable trends. The number of electrons in the outermost shell, or valence electrons, also significantly influences an element's chemical behavior. Elements within a period possess the same number of electron shells but differ in the number of electrons filling those shells.
Trends Across a Period: Unveiling Periodic Properties
As we move across a period from left to right, several key properties exhibit predictable trends. Understanding these trends is vital for predicting chemical reactivity and understanding the behavior of elements.
Atomic Radius: A Gradual Decrease
Atomic radius, the distance from the nucleus to the outermost electron, generally decreases across a period. This is because, while a new electron is added to the same electron shell, the nuclear charge (number of protons) also increases. The increased positive charge pulls the electrons closer to the nucleus, resulting in a smaller atomic radius.
Ionization Energy: A General Increase
Ionization energy is the energy required to remove an electron from a gaseous atom. It generally increases across a period. The stronger nuclear pull caused by the increased positive charge makes it more difficult to remove an electron, thereby increasing ionization energy. Exceptions can occur due to electron shielding and electron-electron repulsion, particularly when dealing with partially filled or fully filled subshells.
Electronegativity: Reflecting Electron Attraction
Electronegativity measures an atom's ability to attract electrons in a chemical bond. It generally increases across a period. The increased nuclear charge enhances an atom's ability to attract shared electrons, leading to higher electronegativity. The noble gases, however, are an exception, exhibiting very low electronegativity due to their stable electron configurations.
Metallic Character: A Transition from Left to Right
Metallic character, the tendency of an element to lose electrons and form positive ions, generally decreases across a period. Elements on the left side of the period are typically highly metallic (alkali and alkaline earth metals), readily losing electrons to achieve a stable electron configuration. As we move towards the right, elements become less metallic, eventually transitioning into nonmetals which prefer to gain electrons.
Periods and Electron Configuration: The Quantum Connection
The organization of the periodic table is deeply intertwined with the electronic configuration of elements. Understanding electron configuration is essential for comprehending the behavior of elements within a period.
Electron Shells and Subshells: Building Blocks of Atoms
Electrons reside in specific energy levels (shells), labeled as n=1, n=2, n=3, etc. Each shell can accommodate a certain number of electrons and consists of subshells (s, p, d, f). The filling of these subshells follows specific rules (Aufbau principle, Hund's rule, Pauli exclusion principle), dictating the electron configuration and ultimately the element's position within the period.
Valence Electrons: The Key to Reactivity
Valence electrons are the electrons in the outermost shell. They are crucial in determining an element's chemical behavior and reactivity. Elements within the same period have the same number of electron shells but differ in the number of valence electrons. This difference in valence electrons is the primary reason why elements in a period display diverse chemical properties.
The Seven Periods: A Closer Look
Let's take a closer look at the individual periods and their characteristic features.
Period 1: The Shortest Period
Period 1 contains only two elements: hydrogen (H) and helium (He). They only have electrons in the first energy level (n=1), which consists of only the 1s subshell. This small size and minimal number of electrons result in unique properties for these elements.
Period 2: The Beginning of p-block Elements
Period 2 introduces the p-block elements, significantly increasing the complexity of chemical behavior. Elements in this period have electrons in the n=1 and n=2 energy levels, including the 2s and 2p subshells. This expansion in electron configurations results in a broader range of chemical properties.
Period 3 to Period 7: Increasing Complexity
Periods 3 through 7 exhibit increasing complexity in their electronic structures and corresponding chemical properties. Each successive period introduces new subshells (d and f subshells), further diversifying the elements' characteristics and behavior. The transition metals, lanthanides, and actinides are all found within these periods, showcasing a vast array of chemical reactivity.
Periods and Chemical Bonding: Interactions and Compounds
The periodic arrangement, specifically the organization into periods, directly impacts the types of chemical bonds elements form.
Ionic Bonding: A Transfer of Electrons
Ionic bonding typically occurs between elements from opposite sides of a period. A highly metallic element from the left readily loses electrons to a highly electronegative element from the right. This transfer of electrons creates ions with opposite charges that attract each other, forming an ionic bond.
Covalent Bonding: Sharing Electrons
Covalent bonding frequently occurs between nonmetals within the same period. These elements share electrons to achieve a stable electron configuration. The strength and characteristics of the bond depend on the electronegativity difference between the atoms.
Practical Applications: Periods and Their Usefulness
The understanding of periods and their associated trends is indispensable in numerous practical applications.
Predicting Chemical Reactions: Using Periodic Trends
The periodic table allows chemists to predict the outcome of chemical reactions based on the elements' positions within a period. Knowledge of electronegativity, ionization energy, and metallic character allows for a qualitative understanding of reaction pathways and product formation.
Materials Science: Designing Novel Materials
Understanding the properties of elements within specific periods is fundamental to materials science. By selecting elements with desired properties, scientists can design new materials with specific applications, from stronger alloys to advanced semiconductors.
Understanding Biological Systems: Essential Elements in Life
Several elements crucial for life (e.g., carbon, oxygen, nitrogen) are positioned within specific periods. Understanding their electronic configurations and chemical behavior provides insight into the fundamental processes that underpin biological systems.
Conclusion: Periods as a Foundation of Chemical Understanding
In conclusion, a horizontal row on the periodic table is called a period. This seemingly simple term represents a fundamental organizational principle with profound implications for understanding chemical behavior. The arrangement of elements within periods, based on their electronic configuration and principal quantum number, reveals predictable trends in atomic size, ionization energy, electronegativity, and metallic character. This understanding forms the basis for predicting chemical reactions, designing new materials, and interpreting biological processes. A solid grasp of periods is essential for anyone seeking a deep understanding of chemistry and its applications in diverse fields.
Latest Posts
Latest Posts
-
What Is The Gcf Of 42 And 28
Mar 06, 2025
-
The Division Of The Nucleus Is Called
Mar 06, 2025
-
Highest Common Factor Of 8 And 16
Mar 06, 2025
-
What Are The Factors For 11
Mar 06, 2025
-
Least Common Multiple Of 12 And 8
Mar 06, 2025
Related Post
Thank you for visiting our website which covers about What Is A Horizontal Row Called On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
