What Is A Conjugated Double Bond
Juapaving
Mar 17, 2025 · 6 min read
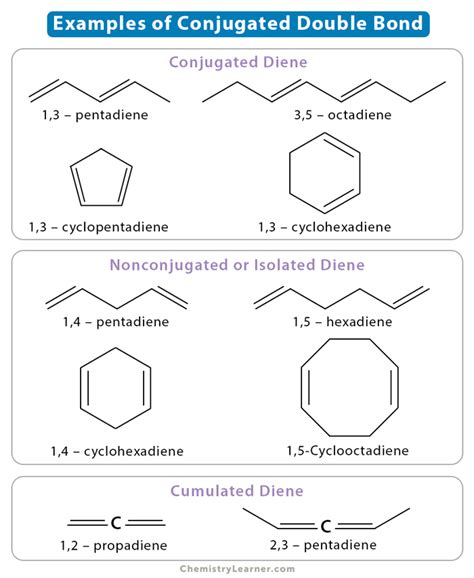
Table of Contents
What is a Conjugated Double Bond? A Deep Dive into Structure, Properties, and Applications
Conjugated double bonds are a fascinating aspect of organic chemistry, impacting the properties and reactivity of molecules in significant ways. Understanding their structure and behavior is crucial for anyone studying or working with organic compounds. This comprehensive guide will delve into the intricacies of conjugated double bonds, exploring their definition, characteristics, spectroscopic properties, and diverse applications across various fields.
Defining Conjugated Double Bonds: The Essence of Delocalization
A conjugated double bond system refers to a molecule containing two or more double bonds separated by one single bond. This specific arrangement allows for significant interaction between the π (pi) electrons of the double bonds, a phenomenon known as conjugation. Crucially, it's this conjugation that differentiates conjugated systems from isolated or cumulated double bonds.
Isolated double bonds: These are double bonds separated by two or more single bonds. There's minimal interaction between the π electrons.
Cumulated double bonds: These are double bonds directly adjacent to each other (e.g., allenes). The interaction is different from conjugation and leads to distinct properties.
The Key Difference: Conjugation leads to a significant delocalization of π electrons. Instead of being confined to individual double bonds, the electrons are spread over the entire conjugated system. This delocalization has profound consequences for the molecule's properties, which we'll examine in detail.
Delocalization: The Driving Force Behind Conjugation's Effects
The delocalization of π electrons in conjugated systems is best visualized using resonance structures. These are multiple Lewis structures that can be drawn for the same molecule, differing only in the placement of electrons. None of these individual resonance structures accurately represent the true structure; instead, the actual structure is a hybrid of all contributing resonance structures.
For example, consider 1,3-butadiene (CH₂=CH-CH=CH₂). We can draw two resonance structures, showing the alternating double and single bonds. However, the true structure is a resonance hybrid where the electron density is spread across all four carbon atoms, resulting in partial double bond character for all the carbon-carbon bonds. This is depicted by the elongated single bonds and shortened double bonds in the resonance hybrid.
This delocalization stabilizes the molecule. The energy of the conjugated system is lower than the energy of an equivalent system with isolated double bonds. This difference in energy is called the delocalization energy or resonance energy.
Spectroscopic Properties: Unveiling Conjugation
Conjugation profoundly impacts the spectroscopic properties of molecules, making spectroscopic techniques invaluable for identifying and characterizing conjugated systems.
UV-Vis Spectroscopy: The Telltale Absorption
Conjugated systems exhibit strong absorption of ultraviolet (UV) and visible (Vis) light. The longer the conjugated system (more double bonds), the longer the wavelength of light absorbed. This is because the energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) decreases with increasing conjugation. This lower energy gap corresponds to absorption at longer wavelengths, often moving absorption from the UV region into the visible region, resulting in colored compounds. This relationship between conjugation length and absorption wavelength is crucial in the design of dyes and pigments.
NMR Spectroscopy: Subtle Chemical Shift Changes
Nuclear Magnetic Resonance (NMR) spectroscopy also provides evidence for conjugation. The chemical shifts of protons and carbon atoms involved in a conjugated system can be slightly different from those in similar non-conjugated systems. These subtle changes are due to the effects of electron delocalization on the local electronic environment of the nuclei.
Reactivity: Electrophilic and Nucleophilic Attacks
The delocalization of electrons in conjugated systems significantly influences their reactivity.
Electrophilic Addition: Regioselectivity and Stereoselectivity
In electrophilic addition reactions, the π electrons of the conjugated system are attacked by an electrophile. The reaction often shows regioselectivity, meaning the electrophile adds to a specific carbon atom, typically the one leading to the most stable carbocation intermediate. Furthermore, the reaction can show stereoselectivity, meaning one stereoisomer is formed preferentially over others.
Nucleophilic Addition: Conjugate Addition
Conjugated systems can also undergo nucleophilic addition reactions, often referred to as conjugate addition or 1,4-addition. In this type of reaction, the nucleophile attacks the carbon atom β to the carbonyl group (or other electron-withdrawing group), resulting in the formation of a new carbon-carbon bond. The reaction proceeds through the formation of a resonance-stabilized enolate intermediate.
Applications: A Wide Range of Industries
The unique properties of conjugated systems make them indispensable across various industries:
Dyes and Pigments: The Colorful World of Conjugation
The ability of conjugated systems to absorb visible light makes them crucial in the synthesis of dyes and pigments. The color and intensity of the color are dependent on the extent of conjugation. Longer conjugated systems absorb at longer wavelengths, leading to deeper colors. Many natural and synthetic dyes owe their vibrant colors to extensive conjugated systems.
Polymers: Strength and Conductivity
Conjugated polymers, also known as conducting polymers, are a fascinating class of materials with unique electrical and optical properties. These polymers contain conjugated π-electron systems along their backbone. The delocalization of electrons allows these polymers to conduct electricity, making them useful in various applications, including flexible electronics, organic light-emitting diodes (OLEDs), and sensors. Examples include polyacetylene and polypyrrole.
Pharmaceuticals: Active Ingredients with Specific Actions
Many biologically active molecules contain conjugated systems. The presence of conjugation can significantly influence the molecules' interactions with biological targets, affecting their efficacy and potency. Many drugs contain conjugated systems as part of their structure, and understanding their impact on drug action is crucial in pharmaceutical design.
Natural Products: Ubiquitous in Nature's Designs
Conjugated systems are found abundantly in nature. Carotenoids, responsible for the vibrant colors of many fruits and vegetables, are examples of natural products with extensive conjugation. These molecules play essential roles in photosynthesis and other biological processes. Other examples include lycopene (tomatoes), β-carotene (carrots), and chlorophyll.
Beyond the Basics: Advanced Concepts
The study of conjugated systems extends beyond the basic concepts presented here. More advanced topics include:
- Cross-conjugation: This refers to a situation where a double bond is conjugated to two other double bonds but these two are not conjugated to each other.
- Cyclic conjugation: Conjugation within ring systems, such as benzene and other aromatic compounds.
- Hückel's rule: This rule predicts whether a cyclic conjugated system will be aromatic (particularly stable) or antiaromatic (particularly unstable).
- Linear polyenes: These are long chains of conjugated double bonds with unique optical and electronic properties.
Conclusion: A Versatile and Essential Aspect of Organic Chemistry
Conjugated double bonds are an essential feature of many organic molecules, significantly impacting their structure, properties, and reactivity. Their ability to delocalize electrons leads to unique spectroscopic properties, specific reactivity patterns, and a diverse range of applications across various scientific and technological fields. Understanding these fundamental concepts is crucial for anyone venturing into the world of organic chemistry, providing the foundation for exploring more complex and exciting aspects of the subject. Further investigation into these advanced topics will undoubtedly deepen your appreciation for the importance and versatility of conjugated double bonds.
Latest Posts
Latest Posts
-
Words That Starts With R And Ends With R
Mar 17, 2025
-
Lcm Of 12 9 And 6
Mar 17, 2025
-
What Are A Group Of Kangaroos Called
Mar 17, 2025
-
Lowest Common Multiple Of 28 And 32
Mar 17, 2025
-
What Are The Factor Pairs Of 30
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is A Conjugated Double Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
