What Happens To An Animal Cell In A Hypertonic Solution
Juapaving
Mar 10, 2025 · 6 min read
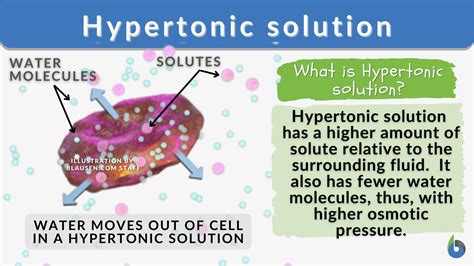
Table of Contents
What Happens to an Animal Cell in a Hypertonic Solution? A Comprehensive Guide
Understanding the behavior of animal cells in different solutions is fundamental to cell biology. This detailed guide delves into the effects of hypertonic solutions on animal cells, exploring the underlying mechanisms, observable changes, and implications for various biological processes. We'll cover the basics, delve into the specifics, and examine the broader context of this crucial concept.
Understanding Osmosis and Tonicity
Before diving into the specific effects of hypertonic solutions, let's establish a clear understanding of osmosis and tonicity. Osmosis is the passive movement of water molecules across a selectively permeable membrane from a region of high water concentration to a region of low water concentration. This movement continues until equilibrium is reached, meaning the water concentration is equal on both sides of the membrane.
Tonicity refers to the relative concentration of solutes in two solutions separated by a selectively permeable membrane. There are three main types of tonicity:
- Isotonic: The solute concentration is equal on both sides of the membrane. There is no net movement of water.
- Hypotonic: The solute concentration is lower outside the cell than inside the cell. Water moves into the cell, potentially causing it to swell and even burst (lysis).
- Hypertonic: The solute concentration is higher outside the cell than inside the cell. This is the focus of this article.
The Fate of an Animal Cell in a Hypertonic Solution
When an animal cell is placed in a hypertonic solution, the concentration of solutes is higher outside the cell than inside. This creates a concentration gradient for water, driving water molecules to move out of the cell across the cell membrane via osmosis. This outward movement of water leads to several significant changes within the cell.
1. Cellular Shrinkage (Plasmolysis): The Primary Effect
The most immediate and noticeable effect is cellular shrinkage, also known as plasmolysis. As water exits the cell, the cytoplasm shrinks and pulls away from the cell membrane. This detachment is clearly visible under a microscope. The extent of shrinkage depends on several factors, including the initial cell size, the concentration of the hypertonic solution, and the duration of exposure.
2. Changes in Cell Volume and Pressure: Impact on Cellular Function
The loss of water significantly reduces the cell's volume and internal pressure (turgor pressure). This decrease in turgor pressure has profound consequences for various cellular functions:
- Reduced Cell Metabolism: Metabolic processes rely on the proper functioning of cellular organelles and enzymes, which are sensitive to changes in cell volume and pressure. Shrinkage can disrupt these processes, leading to a decrease in metabolic activity.
- Impaired Transport Processes: The transport of molecules across the cell membrane, both active and passive, is dependent on the cell's integrity and structural support. Plasmolysis can damage the membrane and disrupt these vital transport mechanisms.
- Compromised Cell Signaling: Cell signaling, essential for communication between cells and coordination of cellular activities, relies on proper cell volume and shape. Shrinkage can interfere with the signaling processes and impact cellular responses.
3. Damage to Cellular Structures: Long-Term Effects
Prolonged exposure to a hypertonic solution can cause significant damage to cellular structures. The cell membrane, which plays a crucial role in maintaining cellular integrity and regulating transport processes, can be severely affected. The shrinkage can lead to:
- Membrane Damage: The cell membrane may become damaged or disrupted, leading to leakage of cellular contents and compromised barrier function. This can result in cell death.
- Organelle Dysfunction: Organelles like mitochondria, responsible for energy production, and the endoplasmic reticulum, involved in protein synthesis, can be affected by changes in cell volume and pressure. This can result in decreased energy production and impaired protein synthesis.
- DNA Damage: In extreme cases, prolonged exposure to hypertonic solutions can lead to damage to the cell's DNA, potentially leading to mutations or cell death.
4. Cell Death (Necrosis or Apoptosis): The Ultimate Consequence
If the cell is unable to adapt to the hypertonic environment, it will eventually undergo cell death. This can occur through two main pathways:
- Necrosis: This is a form of cell death characterized by swelling and rupture of the cell. Necrosis is typically associated with injury or trauma to the cell.
- Apoptosis: This is a programmed form of cell death, a controlled process that eliminates damaged or unwanted cells. In some cases, a cell may initiate apoptosis in response to severe hypertonic stress.
Factors Affecting the Cell's Response
The severity of the effects of a hypertonic solution on an animal cell depends on several factors:
- Concentration of the hypertonic solution: The higher the solute concentration, the greater the water loss and the more pronounced the effects.
- Duration of exposure: Short-term exposure may cause temporary shrinkage, while prolonged exposure can lead to irreversible damage and cell death.
- Type of solute: Different solutes can have different effects on the cell, depending on their permeability and interactions with cellular components.
- Cell type: Different cell types may have varying sensitivities to hypertonic stress, reflecting differences in their membrane properties and ability to regulate water balance.
Examples of Hypertonic Environments and Their Biological Significance
Hypertonic environments are not uncommon in biological systems. Understanding how cells respond to these conditions is vital in various contexts:
- Dehydration: Dehydration in organisms results in hypertonic conditions in the body's fluids, causing cells to lose water. This can have significant effects on organ function and overall health.
- Saline Solutions: Solutions with high salt concentrations are hypertonic and are used in certain medical procedures. The effects of these solutions on cells need careful consideration.
- Food Preservation: Hypertonic solutions, such as high-concentration sugar or salt solutions, are often used to preserve food by creating an environment that inhibits the growth of microorganisms. This works by drawing water out of microbial cells, preventing their growth and survival.
- Marine Environments: Marine organisms face the challenge of living in a hypertonic environment, having developed specialized mechanisms to regulate their water balance and maintain their cellular integrity.
Mechanisms of Cellular Adaptation to Hypertonic Stress
Animal cells have evolved various mechanisms to cope with hypertonic stress. These mechanisms help to minimize water loss and maintain cellular function. Some key adaptive mechanisms include:
- Osmolyte Accumulation: Cells can accumulate compatible osmolytes, which are small organic molecules that don't interfere with cellular processes but increase intracellular solute concentration, helping to reduce water loss.
- Ion Transport: Cells can regulate the transport of ions like sodium and potassium to maintain osmotic balance.
- Aquaporin Regulation: Aquaporins are water channels in the cell membrane. Cells can regulate the number and activity of these channels to control water movement.
- Cellular Remodeling: In some cases, cells may undergo structural changes to withstand hypertonic stress.
Conclusion: The Importance of Understanding Hypertonic Effects
The response of animal cells to hypertonic solutions is a complex phenomenon with significant biological implications. Understanding this process is fundamental to various fields, including medicine, agriculture, and environmental biology. From the effects of dehydration on the human body to the development of food preservation techniques, a thorough grasp of how cells react to hypertonic environments is critical for addressing a wide range of biological challenges. The study of this process continues to uncover new insights into cellular mechanisms and adaptive responses, offering valuable information for advancing scientific knowledge and developing innovative solutions in diverse fields.
Latest Posts
Latest Posts
-
What Is The Prime Factorization 140
Mar 11, 2025
-
In The Figure What Is The Value Of X
Mar 11, 2025
-
What Is Xxviii In Roman Numerals
Mar 11, 2025
-
Distributive Property Calculator Step By Step
Mar 11, 2025
-
Which Of The Following Is Abiotic
Mar 11, 2025
Related Post
Thank you for visiting our website which covers about What Happens To An Animal Cell In A Hypertonic Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
