What Happens To A Substance At Critical Temperatures
Juapaving
Mar 14, 2025 · 7 min read
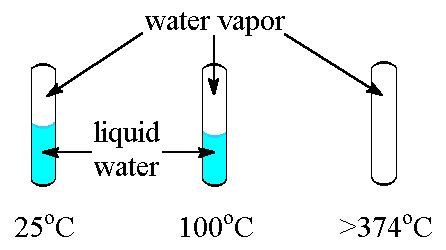
Table of Contents
What Happens to a Substance at Critical Temperatures?
The world of physics holds many fascinating phenomena, and among them, the behavior of substances at their critical temperatures stands out as a particularly intriguing example. Understanding what happens at this point requires delving into the intricacies of phase transitions and the properties of matter under extreme conditions. This article explores the complexities of critical temperatures, providing a comprehensive overview of the changes a substance undergoes and the scientific principles governing this transformative process.
Understanding Phase Transitions and Critical Points
Before diving into the specifics of critical temperatures, it's essential to grasp the concept of phase transitions. Phase transitions refer to the transformations a substance undergoes when subjected to changes in temperature and/or pressure. Familiar examples include the melting of ice (solid to liquid), the boiling of water (liquid to gas), and the deposition of frost (gas to solid). These transitions are often characterized by abrupt changes in physical properties such as density, viscosity, and heat capacity.
A critical point represents the end point of a phase boundary – the specific temperature and pressure at which the distinction between two phases disappears. Beyond this critical point, the substance exists in a supercritical fluid state, possessing properties of both liquids and gases. This state is characterized by unique properties that are distinctly different from either the liquid or gaseous phases.
The Significance of Critical Temperature
The critical temperature (Tc) is the temperature above which a substance cannot exist as a distinct liquid, regardless of the pressure applied. It's a fundamental thermodynamic property specific to each substance and crucial in understanding its behavior under varying conditions. For example, water has a critical temperature of 374°C (647K). Above this temperature, no amount of pressure can liquefy water vapor; it remains a supercritical fluid.
Properties of Substances at Critical Temperatures
At the critical temperature, several dramatic changes occur in the substance's properties:
1. Density and Viscosity Changes:
One of the most striking observations is the gradual disappearance of the density difference between the liquid and gas phases. As the substance approaches its critical temperature, the density of the liquid decreases while the density of the gas increases. At the critical point, these densities become equal, eliminating the distinct liquid-gas interface. Similarly, the viscosity of the substance changes significantly, becoming less distinguishable between liquid and gas phases as the critical temperature is approached.
2. Opalescence and Fluctuations:
As the critical temperature is approached, the substance exhibits a phenomenon known as critical opalescence. This is characterized by intense light scattering due to large-scale density fluctuations within the substance. These fluctuations, essentially tiny regions of varying density, grow in size as the critical point is approached, leading to the milky, cloudy appearance of the substance. This opalescence provides visual evidence of the significant changes in the substance's structure near the critical point.
3. Heat Capacity Divergence:
The heat capacity, the amount of heat required to raise the temperature of a substance by a certain amount, undergoes a significant change near the critical point. It often exhibits a divergence, meaning it approaches infinity as the critical temperature is reached. This implies that an increasingly large amount of energy is required to change the temperature of the substance near its critical point. This behavior reflects the considerable energy required to overcome the strong intermolecular interactions present at these conditions.
4. Loss of Surface Tension:
Another significant change is the disappearance of the surface tension. Surface tension is a result of the imbalance in intermolecular forces at the interface between two phases (e.g., liquid and gas). As the critical temperature is reached, the distinction between phases vanishes, and thus, so does the surface tension. This leads to unique behavior at the interface where typically distinct phases are observed.
5. Supercritical Fluid Behavior:
Beyond the critical point, the substance exists as a supercritical fluid. This state possesses unique properties that make it a versatile solvent and a powerful tool in various applications. Supercritical fluids have densities comparable to liquids, allowing them to dissolve many substances efficiently. However, they also possess the low viscosities and high diffusivities of gases, allowing for rapid penetration and efficient extraction. This combination makes supercritical fluids ideal for applications such as supercritical fluid extraction (SFE) in various industries including food processing and pharmaceuticals.
The Role of Intermolecular Forces
The behavior of substances at critical temperatures is intricately linked to the intermolecular forces acting between the molecules. These forces, including van der Waals forces, hydrogen bonds, and dipole-dipole interactions, dictate the strength of the attractive forces holding the molecules together. As the temperature increases, the kinetic energy of the molecules overcomes these attractive forces, leading to the transition from a more ordered phase (liquid) to a less ordered phase (gas). At the critical point, the kinetic energy is sufficient to completely overcome the intermolecular forces, leading to the formation of the supercritical fluid.
Applications of Critical Temperatures and Supercritical Fluids
The unique properties of supercritical fluids, stemming from their behavior near the critical point, have led to a wide range of applications across various industries:
1. Supercritical Fluid Extraction (SFE):
SFE utilizes supercritical fluids, such as supercritical carbon dioxide (SC-CO2), to extract valuable compounds from various materials. SC-CO2's ability to selectively dissolve specific compounds makes it ideal for extracting essential oils, flavors, and pharmaceuticals without using harsh chemical solvents. The process is environmentally friendly and produces high-quality extracts.
2. Chromatography:
Supercritical fluid chromatography (SFC) is a separation technique that leverages the unique solvating properties of supercritical fluids. SFC offers advantages over traditional liquid chromatography, providing faster separation times and improved resolution. Its applications span diverse fields, including pharmaceutical analysis, environmental monitoring, and food analysis.
3. Chemical Reactions:
Supercritical fluids can act as unique reaction media, altering reaction pathways and improving the efficiency of chemical processes. The tunable properties of supercritical fluids allow for precise control over reaction conditions, leading to enhanced yields and selectivity.
4. Material Processing:
Supercritical fluids are employed in various material processing techniques, including the synthesis of nanoparticles and the preparation of porous materials. Their ability to penetrate and modify material structures makes them ideal for creating novel materials with tailored properties.
Beyond the Basics: Advanced Concepts
The behavior of substances at critical temperatures is a rich field of study, extending beyond the basic concepts discussed so far. Advanced concepts include:
1. Critical Exponents:
The behavior of various thermodynamic properties near the critical point can be described using critical exponents. These exponents are universal constants, meaning they are independent of the specific substance but depend on the type of phase transition. They provide a powerful framework for understanding the scaling behavior of physical quantities near the critical point.
2. Renormalization Group Theory:
Renormalization group theory is a powerful theoretical framework that explains the universality of critical exponents and provides a deeper understanding of the scaling behavior near critical points. It describes how the system's behavior changes under scale transformations, revealing universal properties that apply across various systems.
3. Finite-Size Scaling:
Finite-size scaling considers the effects of system size on the behavior near the critical point. In real-world experiments, the system always has a finite size, which affects the critical behavior and the observed critical exponents. Finite-size scaling allows for correction of the finite-size effects and for obtaining the thermodynamic limit values of critical exponents.
Conclusion:
The study of substances at critical temperatures offers a fascinating glimpse into the complex interplay between temperature, pressure, and intermolecular forces. The unique properties exhibited by substances near and beyond their critical point, particularly the formation of supercritical fluids, have opened up new avenues for technological advancements across diverse fields. From environmentally friendly extraction techniques to innovative material processing methods, the applications of critical temperature phenomena continue to expand, shaping the future of various industries. The ongoing research in this area promises even more exciting discoveries and advancements in our understanding of matter under extreme conditions. Continued exploration of critical phenomena will undoubtedly lead to further innovations and applications in the years to come.
Latest Posts
Latest Posts
-
Common Factors Of 60 And 72
Mar 14, 2025
-
Is Coal And Charcoal The Same Thing
Mar 14, 2025
-
What Is The Multiple Of 7
Mar 14, 2025
-
How Many Faces Are There On A Standard Dice
Mar 14, 2025
-
What Is The Factor Of 24
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about What Happens To A Substance At Critical Temperatures . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
