What Difference Between Heat And Temperature
Juapaving
Mar 12, 2025 · 6 min read
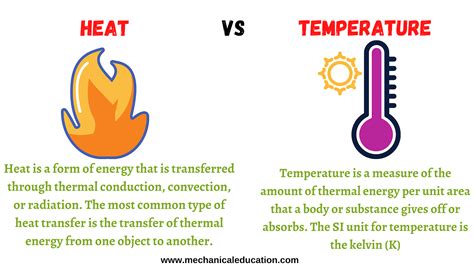
Table of Contents
What's the Difference Between Heat and Temperature?
Heat and temperature are often used interchangeably in everyday conversation, but in the world of physics and thermodynamics, they represent distinct concepts. Understanding the difference is crucial for grasping many scientific phenomena, from weather patterns to the workings of engines. This comprehensive guide will delve into the nuanced distinctions between heat and temperature, exploring their definitions, measurements, and practical applications.
Defining Heat and Temperature: A Fundamental Distinction
Heat, at its core, is a form of energy. It's the total kinetic energy of the moving particles within a substance. These particles – atoms and molecules – are constantly in motion, vibrating, rotating, and translating. The faster these particles move, the more kinetic energy they possess, and the greater the heat content of the substance. Heat is transferred between objects of different temperatures, flowing from hotter objects to colder ones until thermal equilibrium is reached – a state where both objects are at the same temperature. This transfer of energy can occur through conduction, convection, or radiation.
Temperature, on the other hand, is a measure of the average kinetic energy of the particles in a substance. It's a measure of how hot or cold something is. While heat represents the total kinetic energy, temperature reflects the average kinetic energy. This subtle but crucial distinction means that two objects can have the same temperature but vastly different amounts of heat.
Illustrative Example: A Cup of Coffee and a Swimming Pool
Imagine a cup of hot coffee and a large swimming pool filled with lukewarm water. The coffee might be significantly hotter (higher temperature) than the pool water. However, the swimming pool, due to its considerably larger volume and mass, contains far more total kinetic energy (more heat) than the cup of coffee. The pool's greater mass means it has a far greater number of water molecules, even though each molecule has a lower average kinetic energy.
Measuring Heat and Temperature: Units and Scales
Heat is typically measured in joules (J), the standard unit of energy in the International System of Units (SI). Other units, such as calories (cal) and British thermal units (BTU), are also used, but joules provide a unified measure across various energy forms. The amount of heat transferred is dependent on factors like the mass of the substance, its specific heat capacity (the amount of heat required to raise the temperature of 1 gram of a substance by 1 degree Celsius), and the temperature change.
Temperature is measured using various scales, including:
- Celsius (°C): Based on the freezing point (0°C) and boiling point (100°C) of water at standard atmospheric pressure. Widely used internationally.
- Fahrenheit (°F): Used primarily in the United States. Its freezing and boiling points of water are 32°F and 212°F, respectively.
- Kelvin (K): The absolute temperature scale, starting at absolute zero (0 K), the theoretical point where all molecular motion ceases. One Kelvin degree is equal in size to one Celsius degree. Kelvin is the preferred unit in scientific calculations as it avoids negative values.
Heat Transfer Mechanisms: Conduction, Convection, and Radiation
Understanding heat transfer is essential to comprehending how heat moves from one place to another and how temperature changes occur. Three primary mechanisms govern heat transfer:
1. Conduction: Direct Energy Transfer
Conduction is the transfer of heat through direct contact. When a hot object touches a colder object, the faster-moving particles in the hot object collide with the slower-moving particles in the colder object, transferring kinetic energy. This process continues until both objects reach thermal equilibrium. Metals are excellent conductors of heat, while materials like wood and plastic are poor conductors (insulators).
2. Convection: Heat Transfer through Fluids
Convection involves heat transfer through the movement of fluids (liquids or gases). As a fluid heats up, its density decreases, causing it to rise. Cooler, denser fluid then sinks to replace it, creating a cycle of movement that distributes heat. Examples of convection include boiling water and the formation of weather patterns.
3. Radiation: Heat Transfer through Electromagnetic Waves
Radiation is the transfer of heat through electromagnetic waves, which can travel through a vacuum. The sun's heat reaches the Earth through radiation. All objects emit thermal radiation, with hotter objects emitting more energy at shorter wavelengths. This is why we feel heat from a fireplace or a burning candle.
Specific Heat Capacity: A Key Factor in Temperature Change
Specific heat capacity is a crucial property that describes a substance's resistance to temperature changes. It represents the amount of heat required to raise the temperature of one unit mass of the substance by one degree. Substances with high specific heat capacity require a large amount of heat to increase their temperature, while substances with low specific heat capacity heat up quickly. Water, for example, has a remarkably high specific heat capacity, making it an excellent coolant.
Heat Capacity and Latent Heat: Phase Changes
Beyond specific heat capacity, the concept of heat capacity encompasses the total amount of heat required to change the temperature of an object. When a substance undergoes a phase change (e.g., melting ice, boiling water), its temperature remains constant despite the addition or removal of heat. The heat energy involved in these phase transitions is called latent heat. Latent heat of fusion refers to the heat required to melt a solid, while latent heat of vaporization refers to the heat required to vaporize a liquid.
Applications of Heat and Temperature: From Everyday Life to Advanced Technology
The concepts of heat and temperature are fundamental to a wide range of applications, spanning various fields:
- Meteorology: Understanding heat transfer processes is critical for weather forecasting and climate modeling.
- Engineering: Designing efficient engines, power plants, and HVAC systems relies on the principles of thermodynamics and heat transfer.
- Cooking: Controlling heat is essential for preparing food, with different cooking methods relying on different heat transfer mechanisms.
- Medicine: Maintaining body temperature is crucial for human health, and understanding heat transfer is important in medical therapies.
- Materials Science: The properties of materials often depend heavily on temperature, requiring careful control during manufacturing processes.
- Industrial Processes: Many industrial processes, such as metal casting and chemical reactions, involve precise control of heat and temperature.
Conclusion: A Clear Understanding of Heat and Temperature
The distinction between heat and temperature is more than just a semantic difference; it's a fundamental concept in physics with profound implications across numerous scientific disciplines and technological applications. While often used interchangeably in everyday language, understanding that heat represents the total kinetic energy of a system, while temperature reflects its average kinetic energy, is vital for a comprehensive understanding of thermal phenomena. Mastering the concepts of heat transfer, specific heat capacity, and latent heat provides the foundation for tackling a broad spectrum of challenges in science and engineering. This knowledge is essential for innovation and problem-solving in a wide variety of fields, from developing more efficient energy systems to advancing medical treatments. Remember, the seemingly simple concepts of heat and temperature are the building blocks of a much larger and fascinating world of thermodynamics.
Latest Posts
Latest Posts
-
A Food Chain Starts With A
May 09, 2025
-
Which Of The Following Statements About Chlorophyll Is Correct
May 09, 2025
-
Kinetic Energy Is Energy An Object Has Because Of Its
May 09, 2025
-
What Is The Least Common Multiple Of 20 And 40
May 09, 2025
-
48 Inches Is What In Feet
May 09, 2025
Related Post
Thank you for visiting our website which covers about What Difference Between Heat And Temperature . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.