What Are The Polymers Of Proteins
Juapaving
Mar 20, 2025 · 7 min read
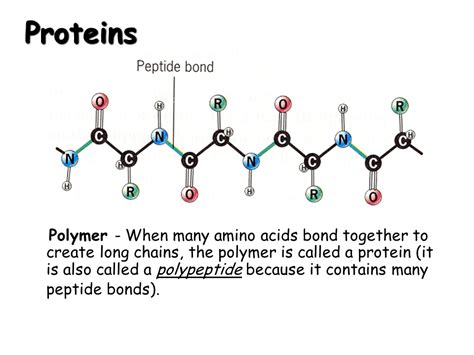
Table of Contents
What Are the Polymers of Proteins? Understanding Amino Acids and Peptide Bonds
Proteins are the workhorses of the cell, crucial for virtually every biological process imaginable. From catalyzing reactions (enzymes) to providing structural support (collagen), their diverse functions stem from their incredibly complex structures. But what are these structures actually made of? The answer lies in understanding the polymers of proteins: amino acids linked together by peptide bonds.
Amino Acids: The Building Blocks of Proteins
Proteins are polymers, meaning they are large molecules composed of repeating subunits. These subunits, in the case of proteins, are amino acids. There are 20 different standard amino acids that are commonly found in proteins, each with a unique side chain (also known as an R-group) that determines its properties. These properties—size, charge, polarity, hydrophobicity—dictate how a protein folds and functions.
The General Structure of an Amino Acid
Each amino acid shares a common basic structure:
- Amino group (-NH₂): This is a basic group, meaning it tends to accept protons (H⁺).
- Carboxyl group (-COOH): This is an acidic group, meaning it tends to donate protons (H⁺).
- Central carbon atom (α-carbon): This carbon atom is bonded to the amino group, the carboxyl group, a hydrogen atom, and the R-group.
- R-group (side chain): This is the variable part of the amino acid, and it's what makes each amino acid unique. The R-group can be anything from a simple hydrogen atom (as in glycine) to a complex aromatic ring (as in tryptophan).
Classification of Amino Acids Based on R-Group Properties
Amino acids are frequently categorized based on the properties of their R-groups:
-
Nonpolar, aliphatic amino acids: These amino acids have hydrocarbon side chains that are hydrophobic (water-repelling). Examples include glycine, alanine, valine, leucine, isoleucine, and methionine.
-
Aromatic amino acids: These amino acids have side chains containing aromatic rings. They are generally hydrophobic, although some can participate in weak interactions with water molecules. Examples include phenylalanine, tyrosine, and tryptophan.
-
Polar, uncharged amino acids: These amino acids have side chains that are hydrophilic (water-attracting) but do not carry a net charge at physiological pH. Examples include serine, threonine, cysteine, asparagine, and glutamine.
-
Positively charged amino acids (basic amino acids): These amino acids have side chains with a positive charge at physiological pH. Examples include lysine, arginine, and histidine.
-
Negatively charged amino acids (acidic amino acids): These amino acids have side chains with a negative charge at physiological pH. Examples include aspartic acid and glutamic acid.
The properties of these R-groups are absolutely crucial in determining the protein's three-dimensional structure and, consequently, its function. For instance, hydrophobic R-groups tend to cluster together in the protein's interior, away from the aqueous environment of the cell. Charged R-groups may participate in ionic interactions with other charged groups, while polar R-groups can form hydrogen bonds with water molecules or other polar groups.
Peptide Bonds: Linking Amino Acids Together
Amino acids are linked together by peptide bonds, which are covalent bonds formed between the carboxyl group of one amino acid and the amino group of another. This reaction is a dehydration synthesis, meaning that a water molecule is released during the bond formation.
The Peptide Bond Formation
The formation of a peptide bond involves a nucleophilic attack by the nitrogen atom of the amino group on the carbonyl carbon of the carboxyl group. This process is energetically unfavorable and requires enzymatic catalysis in the ribosome during protein synthesis. The resulting peptide bond exhibits a partial double-bond character due to resonance, which restricts rotation around the bond and influences protein conformation.
Peptide Chains and Polypeptides
The result of this bond formation is a dipeptide (two amino acids linked together), then a tripeptide (three amino acids), and so on. Longer chains of amino acids are called polypeptides. When a polypeptide chain folds into a specific three-dimensional structure and performs a biological function, it is then classified as a protein. The length of a polypeptide chain can vary enormously, from a few dozen amino acids to thousands.
Levels of Protein Structure: From Primary to Quaternary
The structure of a protein dictates its function. Protein structure is often described in terms of four levels of organization:
1. Primary Structure: The Amino Acid Sequence
The primary structure of a protein is simply the linear sequence of amino acids in the polypeptide chain. This sequence is dictated by the genetic code, which specifies the order in which amino acids are added during protein synthesis. Even a single amino acid change in the primary structure can have profound effects on the protein's function, as seen in sickle cell anemia, caused by a single amino acid substitution in the hemoglobin protein.
2. Secondary Structure: Local Folding Patterns
The secondary structure refers to local folding patterns within the polypeptide chain, stabilized by hydrogen bonds between the backbone amide and carbonyl groups. Common secondary structures include:
-
α-helices: These are right-handed coiled structures, stabilized by hydrogen bonds between the carbonyl oxygen of one amino acid and the amide hydrogen of an amino acid four residues further down the chain.
-
β-sheets: These are extended structures formed by hydrogen bonding between adjacent polypeptide chains (or segments of the same chain). β-sheets can be parallel (chains run in the same direction) or antiparallel (chains run in opposite directions).
-
Turns and loops: These less-ordered structures connect α-helices and β-sheets, providing flexibility in the overall protein structure.
3. Tertiary Structure: The Three-Dimensional Arrangement
The tertiary structure represents the overall three-dimensional arrangement of a polypeptide chain, including all its secondary structures. This structure is determined by a variety of interactions between the R-groups of the amino acids:
- Hydrophobic interactions: Hydrophobic R-groups cluster together in the protein's interior, away from water.
- Hydrogen bonds: These form between polar R-groups.
- Ionic bonds (salt bridges): These occur between oppositely charged R-groups.
- Disulfide bonds: These covalent bonds form between cysteine residues, strongly stabilizing the tertiary structure.
The tertiary structure is crucial for protein function. The precise three-dimensional arrangement of amino acid residues creates a specific binding site for other molecules, allowing the protein to carry out its biological function.
4. Quaternary Structure: Multiple Polypeptide Chains
Some proteins consist of multiple polypeptide chains (subunits) that associate to form a functional unit. The arrangement of these subunits constitutes the quaternary structure. The interactions between subunits are similar to those that stabilize tertiary structure—hydrophobic interactions, hydrogen bonds, ionic bonds, and disulfide bonds. Examples of proteins with quaternary structure include hemoglobin and many enzymes.
Factors Affecting Protein Structure and Function
Several factors can influence a protein's structure and consequently, its function:
-
Temperature: High temperatures can disrupt the weak interactions (hydrogen bonds, ionic bonds) that stabilize protein structure, leading to denaturation (unfolding) of the protein.
-
pH: Changes in pH can alter the charges on amino acid R-groups, affecting ionic interactions and protein structure.
-
Salinity: High salt concentrations can disrupt ionic interactions and hydrogen bonds, affecting protein structure.
-
Reducing agents: These agents can break disulfide bonds, leading to protein unfolding.
Conclusion: The Intricate World of Protein Polymers
The polymers of proteins, amino acids linked by peptide bonds, are the fundamental building blocks of life. The remarkable diversity of protein functions arises from the vast number of possible amino acid sequences and the complex folding patterns that these sequences adopt. Understanding the structure of proteins – from the primary sequence to the quaternary arrangement – is crucial for comprehending their biological roles and developing strategies to manipulate them for various applications, ranging from medicine to biotechnology. The ongoing research into protein structure and function continues to unravel the intricate details of this fascinating field, revealing new insights into the fundamental processes of life.
Latest Posts
Latest Posts
-
Electronic Banking Is Also Known As
May 09, 2025
-
What Is A Group Of Tissues Working Together Called
May 09, 2025
-
Part Of The Flower That Makes Pollen
May 09, 2025
-
Newtons Second Law Of Motion Examples In Everyday Life
May 09, 2025
-
Most Reactive Metal On The Periodic Table
May 09, 2025
Related Post
Thank you for visiting our website which covers about What Are The Polymers Of Proteins . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.