What Are The Horizontal Rows On A Periodic Table Called
Juapaving
Mar 21, 2025 · 6 min read
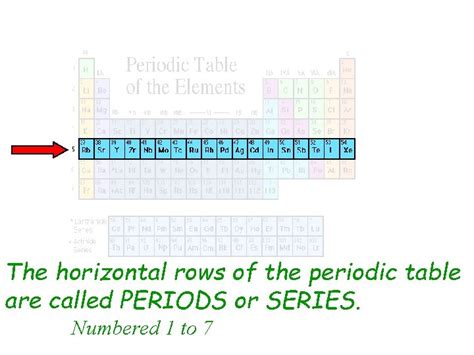
Table of Contents
What Are the Horizontal Rows on a Periodic Table Called? A Deep Dive into Periods
The periodic table, that iconic grid of elements, is a cornerstone of chemistry. Understanding its structure is crucial to grasping the fundamental properties and relationships between different elements. One of the most basic, yet often overlooked, aspects is the identification of its horizontal rows. This article dives deep into the answer to the question: What are the horizontal rows on a periodic table called? They are called periods. But understanding why they're called periods and what they signify goes far beyond a simple definition.
Understanding Periods: More Than Just Horizontal Rows
The horizontal rows on the periodic table are known as periods. Each period represents a principal energy level or shell within an atom. This means that the elements within a given period have their outermost electrons filling the same principal energy level. As you move across a period from left to right, the atomic number increases, meaning the number of protons and electrons in the atom also increases. This systematic addition of electrons leads to predictable trends in atomic properties.
The Significance of Period Number
The period number corresponds directly to the highest principal quantum number (n) of the electrons in an atom's ground state. For example, all elements in Period 1 (hydrogen and helium) have electrons with a principal quantum number of n=1. Similarly, Period 2 elements have electrons with n=2, Period 3 elements have n=3, and so on. This directly relates to the energy level and distance of the outermost electrons from the nucleus.
Period Length and Electron Shells
The length of each period isn't uniform. The number of elements in a period is determined by the number of electrons that can occupy the subshells within a given principal energy level. The specific number of elements in each period is governed by the quantum mechanical rules that dictate electron configurations.
-
Period 1: This is the shortest period, containing only two elements: hydrogen (H) and helium (He). This is because the first principal energy level (n=1) only has one subshell, the 1s subshell, which can accommodate a maximum of two electrons.
-
Period 2 and 3: These periods each have eight elements. This is because the second and third principal energy levels have four subshells (2s, 2p; 3s, 3p), each capable of holding a specific number of electrons (s subshells hold 2 and p subshells hold 6). The filling of these subshells explains the octet rule, often seen in the chemistry of these elements.
-
Period 4 and 5: These periods have 18 elements each. This increase is due to the addition of the d subshells (3d and 4d respectively). The d subshells can hold up to 10 electrons, which accounts for the expansion in period length.
-
Period 6: Period 6 has 32 elements. This is due to the addition of the f subshells (4f), which can accommodate up to 14 electrons.
-
Period 7: This period is currently incomplete, with only some elements synthesized artificially. The pattern would continue with the addition of more elements as the 5g subshell is filled.
Trends Across a Period: Atomic Properties
The arrangement of elements within a period reflects systematic changes in their atomic properties. Moving across a period from left to right:
-
Atomic Radius Decreases: As the number of protons increases, the positive charge in the nucleus increases. This stronger pull on the electrons results in a smaller atomic radius.
-
Ionization Energy Increases: Ionization energy is the energy needed to remove an electron from an atom. As the nuclear charge increases, it becomes more difficult to remove an electron, leading to a higher ionization energy.
-
Electronegativity Increases: Electronegativity is the ability of an atom to attract electrons in a chemical bond. As the nuclear charge increases across a period, the atom's attraction for electrons in a bond increases.
-
Metallic Character Decreases: Metallic properties, such as conductivity and malleability, generally decrease as you move across a period. Elements on the left side of the period are typically metals, while those on the right are nonmetals.
-
Electron Affinity Generally Increases: Electron affinity is the energy change that occurs when an electron is added to a neutral atom. While there are some exceptions, there's a general trend of increasing electron affinity across a period.
The Relationship Between Periods and Groups
Periods and groups (vertical columns) are the two fundamental ways to organize elements on the periodic table. While periods show trends based on electron shells, groups highlight elements sharing similar chemical properties due to the same number of valence electrons (outermost electrons). Understanding both is critical for predicting an element's behavior. The combination of group and period placement provides a powerful predictive tool for chemical reactivity and physical properties.
Exceptions and Irregularities
While the periodic table is organized based on predictable patterns, exceptions and irregularities do exist. These deviations are primarily due to the complex interactions between electrons and the subtleties of electron configurations. Certain electron subshells have slightly different energies, which can lead to variations in atomic properties that don’t strictly follow the general trends across a period.
The Importance of Understanding Periods in Chemistry
Understanding periods is fundamental to many aspects of chemistry, including:
-
Predicting Chemical Reactions: The placement of an element within a period provides crucial insights into its reactivity and how it might interact with other elements.
-
Analyzing Chemical Bonding: Understanding electron configurations and electron shells helps explain the formation of different types of chemical bonds.
-
Explaining Physical Properties: The trends in atomic radii, ionization energy, and electronegativity across a period help account for the differences in the physical properties of elements.
-
Developing New Materials: The periodic table, and the understanding of periods and groups, is a key tool in materials science, aiding in the design and development of new materials with specific desired properties.
Beyond the Basics: Advanced Concepts Related to Periods
The concept of periods extends into more advanced chemical concepts:
-
Quantum Mechanics: The very structure of the periodic table is derived from the principles of quantum mechanics, which describe the behavior of electrons in atoms.
-
Spectroscopy: Analyzing the spectral lines of elements provides experimental confirmation of the electron shell structure and validates the concept of periods.
-
Nuclear Chemistry: While primarily focused on the nucleus, nuclear chemistry also considers the electronic structure of isotopes, linking back to the arrangement of elements within periods.
Conclusion: Periods – A Foundation of Chemical Understanding
In conclusion, the horizontal rows on the periodic table are called periods. This simple term encompasses a profound understanding of atomic structure and chemical behavior. The arrangement of elements within a period reflects the systematic filling of electron shells, leading to predictable trends in atomic properties. Understanding periods is therefore not just a matter of memorization but a key to comprehending the fundamental principles that govern the world of chemistry and beyond. From predicting reactions to designing new materials, the knowledge of periods serves as a cornerstone for scientific inquiry and technological advancement. The seemingly simple horizontal rows represent a fundamental and powerful organizational principle that underpins much of our understanding of the elements and their behavior.
Latest Posts
Latest Posts
-
How Many Litres Is 50 Gallons
Mar 21, 2025
-
Which Of The Following Is Not Possible
Mar 21, 2025
-
How Tall Is 40 Inches In Feet
Mar 21, 2025
-
A Raw Egg Is Fried Physical Or Chemical Change
Mar 21, 2025
-
What Is The Square Root Of 10000
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Are The Horizontal Rows On A Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
