What Are The Conjugate Base And Conjugate Acid Of H2po4-
Juapaving
May 12, 2025 · 6 min read
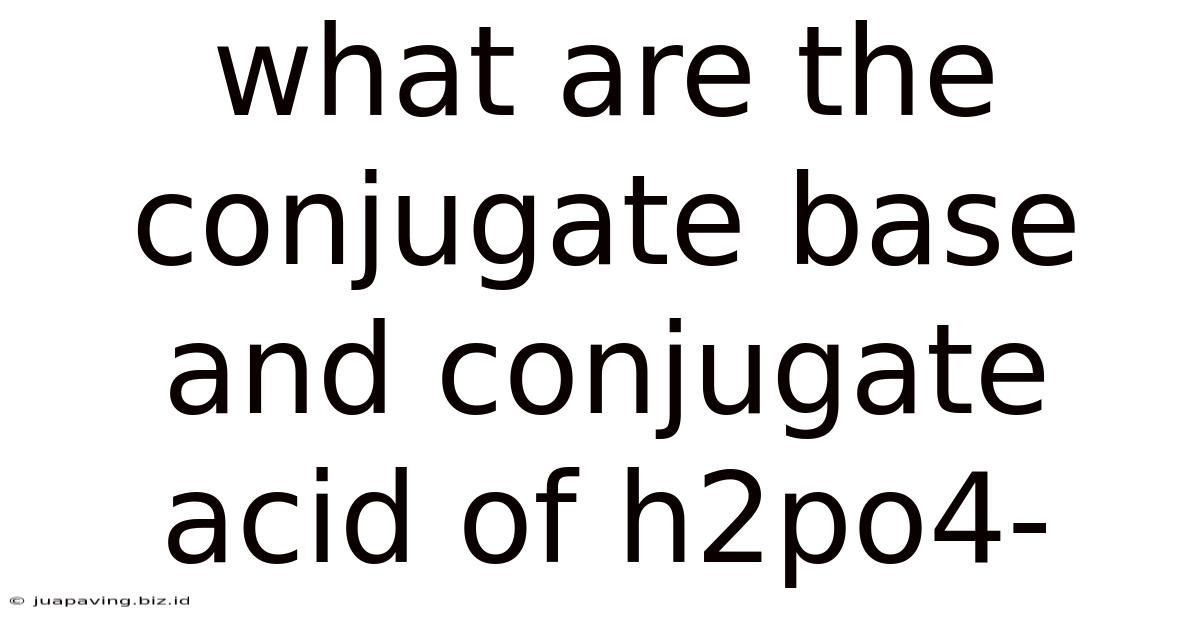
Table of Contents
What are the Conjugate Base and Conjugate Acid of H₂PO₄⁻? A Deep Dive into Acid-Base Chemistry
Understanding conjugate acid-base pairs is fundamental to grasping acid-base chemistry. This article delves deep into the concept, focusing specifically on the dihydrogen phosphate ion, H₂PO₄⁻, exploring its conjugate acid and conjugate base, and examining their properties and roles in various chemical contexts. We'll also touch upon the broader implications within buffer solutions and biological systems.
Understanding Conjugate Acid-Base Pairs
According to the Brønsted-Lowry acid-base theory, an acid is a substance that donates a proton (H⁺), while a base is a substance that accepts a proton. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. These pairs are linked by the difference of a single proton.
The relationship can be represented generally as:
HA ⇌ H⁺ + A⁻
Where:
- HA is the acid
- H⁺ is the proton
- A⁻ is the conjugate base
This equilibrium shows that an acid can donate a proton to become its conjugate base, and the conjugate base can accept a proton to revert back to the acid. The position of the equilibrium depends on the relative strengths of the acid and its conjugate base.
H₂PO₄⁻: A Versatile Amphoteric Ion
Dihydrogen phosphate, H₂PO₄⁻, is a particularly interesting ion because it's amphoteric. This means it can act as both an acid and a base, depending on the chemical environment. This amphoteric nature stems from its ability to both donate and accept protons.
H₂PO₄⁻ as an Acid
When H₂PO₄⁻ acts as an acid, it donates a proton (H⁺) to a base, forming its conjugate base. This reaction can be represented as:
H₂PO₄⁻(aq) ⇌ H⁺(aq) + HPO₄²⁻(aq)
In this reaction:
- H₂PO₄⁻ is the acid. It donates a proton.
- HPO₄²⁻ is the conjugate base. It's what remains after H₂PO₄⁻ loses a proton.
The strength of H₂PO₄⁻ as an acid is moderate, meaning it doesn't completely dissociate in water. The equilibrium lies significantly towards the reactants, indicating that a substantial amount of H₂PO₄⁻ remains undissociated.
H₂PO₄⁻ as a Base
Conversely, H₂PO₄⁻ can also act as a base, accepting a proton from an acid. This reaction can be expressed as:
H₂PO₄⁻(aq) + H⁺(aq) ⇌ H₃PO₄(aq)
In this reaction:
- H₂PO₄⁻ is the base. It accepts a proton.
- H₃PO₄ is the conjugate acid. It's formed when H₂PO₄⁻ gains a proton.
This shows that H₂PO₄⁻ can accept a proton to form phosphoric acid, H₃PO₄. Again, the strength of H₂PO₄⁻ as a base is also moderate. The equilibrium lies somewhat towards the reactants, indicating that a significant portion of H₂PO₄⁻ remains unreacted.
The Properties of H₃PO₄ (Phosphoric Acid) and HPO₄²⁻ (Hydrogen Phosphate)
Understanding the properties of the conjugate acid and base of H₂PO₄⁻ is crucial for comprehending its behavior in different chemical systems.
H₃PO₄ (Phosphoric Acid): The Conjugate Acid
Phosphoric acid (H₃PO₄) is a relatively weak triprotic acid. "Triprotic" signifies that it can donate three protons in successive steps. Each deprotonation step has a different acid dissociation constant (Ka), reflecting the differing ease with which each proton is released. Phosphoric acid is commonly used in food and beverage industries, as well as in fertilizers. Its relatively weak acidity makes it suitable for many applications where a strong acid would be too corrosive or damaging.
Key Properties of H₃PO₄:
- Weak Acid: Does not fully dissociate in water.
- Triprotic: Donates three protons sequentially.
- Moderately Soluble: Dissolves readily in water.
- Non-toxic (at low concentrations): Used extensively in food products.
HPO₄²⁻ (Hydrogen Phosphate): The Conjugate Base
Hydrogen phosphate (HPO₄²⁻) is the conjugate base of H₂PO₄⁻. It's also amphoteric, meaning it can act as both an acid and a base. However, its acidic properties are weaker than those of H₂PO₄⁻. The basic properties of HPO₄²⁻ are more pronounced than its acidic properties. It's an important component in many biological systems, particularly in buffering solutions.
Key Properties of HPO₄²⁻:
- Weak Acid/Weak Base: Exhibits both acidic and basic properties.
- Amphoteric: Can donate and accept a proton.
- Important in Biological Systems: Plays a crucial role in maintaining pH balance.
The Role of H₂PO₄⁻, H₃PO₄, and HPO₄²⁻ in Buffer Solutions
The amphoteric nature of H₂PO₄⁻ makes it exceptionally useful in creating buffer solutions. A buffer solution resists changes in pH upon the addition of small amounts of acid or base. The H₂PO₄⁻/HPO₄²⁻ buffer system is particularly important in biological systems, where maintaining a stable pH is critical for the proper functioning of enzymes and other biomolecules.
The buffer solution works by neutralizing added acids and bases. When a strong acid is added, the HPO₄²⁻ reacts with the added H⁺ ions to form H₂PO₄⁻, minimizing the change in pH. Conversely, when a strong base is added, the H₂PO₄⁻ reacts with the added OH⁻ ions to form HPO₄²⁻ and water, again minimizing the pH change. This ability to resist pH changes is essential for maintaining a stable environment within cells and other biological systems.
H₂PO₄⁻ in Biological Systems
The phosphate system, including H₂PO₄⁻, HPO₄²⁻ and H₃PO₄, plays a vital role in many biological processes. Its significance stems from several key functions:
-
pH Regulation: As discussed, the H₂PO₄⁻/HPO₄²⁻ buffer system is crucial for maintaining intracellular pH homeostasis. This is vital for enzyme activity and overall cellular function.
-
Energy Transfer: Phosphate groups are central to ATP (adenosine triphosphate), the primary energy currency of cells. The transfer of phosphate groups between molecules is a key mechanism for energy storage and release.
-
DNA and RNA Structure: Phosphate groups form the backbone of DNA and RNA molecules, providing structural integrity and contributing to their functionality.
-
Signal Transduction: Phosphate groups are involved in various signal transduction pathways, acting as "on/off" switches for cellular processes.
Conclusion: A Comprehensive Understanding of H₂PO₄⁻ and its Conjugates
The dihydrogen phosphate ion, H₂PO₄⁻, is a fascinating and highly versatile chemical species. Its amphoteric nature, ability to act as both an acid and a base, is key to its roles in buffer solutions and biological systems. By understanding the properties of its conjugate acid (H₃PO₄) and conjugate base (HPO₄²⁻), we can better appreciate its importance in maintaining pH balance, energy transfer, and structural integrity within biological systems. This comprehensive understanding highlights the significance of conjugate acid-base pairs in chemistry and their impact across a wide range of applications. Further research into the intricacies of phosphate chemistry continues to unveil new insights into its crucial roles in biological and chemical processes.
Latest Posts
Latest Posts
-
Action Words That Start With G
May 12, 2025
-
The Atomic Number Of Phosphorus Is
May 12, 2025
-
What Ratios Are Equivalent To 3 4
May 12, 2025
-
Does Phosphorus Have A Gaseous Phase
May 12, 2025
-
What Does The Liver Do In A Frog
May 12, 2025
Related Post
Thank you for visiting our website which covers about What Are The Conjugate Base And Conjugate Acid Of H2po4- . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.