Water Is A Element Compound Or Mixture
Juapaving
Mar 10, 2025 · 5 min read
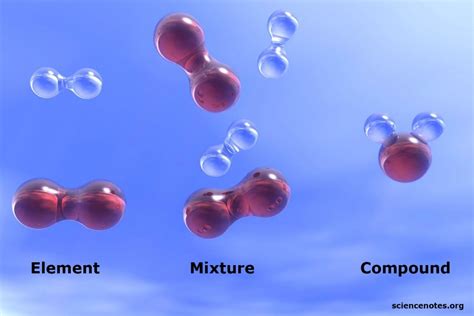
Table of Contents
Water: Element, Compound, or Mixture? Unraveling the Nature of H₂O
The question of whether water is an element, compound, or mixture is a fundamental one in chemistry, often encountered early in scientific education. While seemingly straightforward, a complete understanding requires delving into the definitions of these terms and exploring the unique properties of water. This article will comprehensively examine the nature of water, clarifying its classification and highlighting its significance in various contexts.
Defining the Terms: Element, Compound, and Mixture
Before classifying water, let's establish clear definitions for each term:
Element: An element is a pure substance consisting only of atoms that all have the same number of protons in their atomic nuclei. Elements are the fundamental building blocks of all matter and cannot be broken down into simpler substances by chemical means. Examples include oxygen (O), hydrogen (H), iron (Fe), and gold (Au). The periodic table organizes and displays all known elements.
Compound: A compound is a pure substance formed when two or more different elements chemically combine in a fixed ratio. This combination involves the sharing or transfer of electrons between atoms, forming chemical bonds. Compounds have unique properties that differ from the properties of their constituent elements. For instance, sodium (Na), a highly reactive metal, and chlorine (Cl), a toxic gas, combine to form sodium chloride (NaCl), or table salt, a relatively inert and edible compound. Compounds can be broken down into simpler substances through chemical reactions.
Mixture: A mixture is a combination of two or more substances that are not chemically bonded. The substances in a mixture retain their individual properties and can be separated by physical methods, such as filtration, distillation, or evaporation. Mixtures can be homogeneous (uniform throughout, like saltwater) or heterogeneous (non-uniform, like sand and water).
Water's Chemical Composition: A Compound, Not an Element or Mixture
Water (H₂O) is unequivocally a compound. It's formed by the chemical combination of two elements: hydrogen (H) and oxygen (O). Specifically, two hydrogen atoms covalently bond with one oxygen atom, sharing electrons to achieve a stable electron configuration. This chemical bond gives water its unique properties, distinct from those of hydrogen and oxygen.
Hydrogen, a highly flammable gas, and oxygen, a crucial component of air and vital for respiration, individually possess drastically different characteristics. Their combination into water creates a substance essential for life, possessing properties quite unlike either component. This transformation underscores the concept of a chemical compound – a new substance formed through a chemical reaction, possessing distinct properties from its constituent elements.
The fixed ratio of hydrogen to oxygen in water (2:1) is another defining characteristic of a compound. Every water molecule always consists of two hydrogen atoms and one oxygen atom. This consistent composition differentiates compounds from mixtures, which can have variable ratios of their components.
The Properties of Water: A Testament to its Compound Nature
Many of water's unique properties are a direct consequence of its molecular structure and the nature of the covalent bonds between hydrogen and oxygen. These properties are critical for supporting life on Earth:
-
High Specific Heat Capacity: Water can absorb a large amount of heat with only a small temperature change. This property moderates Earth's climate and helps maintain stable temperatures in aquatic environments.
-
High Heat of Vaporization: Water requires a significant amount of energy to transition from liquid to gas (vapor). This property is crucial for evaporative cooling, such as sweating in animals, which helps regulate body temperature.
-
Universal Solvent: Water's polarity, due to the slightly positive hydrogen atoms and slightly negative oxygen atom, allows it to dissolve many ionic and polar substances, making it an excellent solvent for various biological and chemical processes.
-
Cohesion and Adhesion: Water molecules strongly attract each other (cohesion) due to hydrogen bonding, leading to surface tension and capillary action. Water molecules also adhere to other substances (adhesion), facilitating the movement of water in plants and contributing to various biological functions.
-
Density Anomaly: Ice is less dense than liquid water, causing it to float. This anomaly is crucial for aquatic life, as floating ice insulates the water below, preventing it from freezing solid and allowing aquatic organisms to survive in winter.
Debunking the Mixture Misconception: Why Water Isn't a Mixture
Sometimes, confusion arises because water can exist as a mixture with other substances. For example, saltwater is a mixture of water and sodium chloride. However, the water itself remains a compound within the mixture. The salt dissolves in the water, meaning the sodium and chloride ions are surrounded by water molecules, but the water molecules themselves retain their chemical structure as H₂O. The separation of salt from water can be achieved by physical means, like evaporation, further demonstrating that saltwater is a mixture, not a compound. The water component, however, remains chemically unchanged during this separation.
Water's Importance in Various Fields
Water's unique properties make it essential across diverse fields:
-
Biology: Water is the medium for life, acting as a solvent, transport medium, and participant in countless biochemical reactions.
-
Chemistry: Water serves as a solvent in countless chemical reactions, both in laboratory settings and industrial processes.
-
Physics: Water's properties are studied to understand fluid dynamics, thermodynamics, and other physical phenomena.
-
Engineering: Water's role in hydraulic systems, cooling systems, and many other engineering applications is paramount.
-
Geology: Water plays a crucial role in erosion, weathering, and the formation of geological features.
Conclusion: Water – A Compound Essential for Life
In conclusion, water (H₂O) is undeniably a compound, not an element or a mixture. Its chemical composition, a fixed ratio of hydrogen and oxygen atoms bonded through covalent bonds, and its unique properties, stemming from its molecular structure, firmly place it in the category of compounds. While water can be part of various mixtures, the water molecule itself remains a compound, unchanged in its chemical structure. The distinctive properties of water, stemming from its compound nature, make it an indispensable substance for life and crucial for countless applications in various fields of science and engineering. Understanding water's fundamental nature as a compound allows us to appreciate its critical role in shaping our world and sustaining life as we know it.
Latest Posts
Latest Posts
-
Identify The Meso Isomer Of The Following Compound
May 09, 2025
-
All Events That Occur During One Heartbeat
May 09, 2025
-
What Are The Radioactive Elements In The Periodic Table
May 09, 2025
-
How To Balance C8h18 O2 Co2 H2o
May 09, 2025
-
How Are The Desert And Tundra Similar
May 09, 2025
Related Post
Thank you for visiting our website which covers about Water Is A Element Compound Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.