Vertical Columns On The Periodic Table Are Called
Juapaving
Mar 15, 2025 · 7 min read
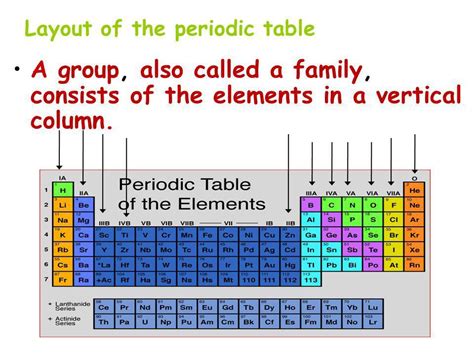
Table of Contents
Vertical Columns on the Periodic Table are Called Groups (or Families)
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding its structure is key to grasping the fundamental principles of chemistry. A common question for students and enthusiasts alike is: what are the vertical columns on the periodic table called? The answer is groups, also known as families. These columns represent elements with similar outer electron shell configurations, leading to shared chemical properties and predictable behaviors. This article delves deep into the world of groups, exploring their characteristics, significance, and the fascinating trends they reveal.
Understanding the Organization of the Periodic Table
The periodic table isn't just a random arrangement of elements. Its organization reflects the underlying structure of atoms. Elements are arranged in order of increasing atomic number (the number of protons in the atom's nucleus). This arrangement reveals a recurring pattern of properties, hence the term "periodic." The horizontal rows are called periods, while the vertical columns are the groups or families.
Periods: Trends Across the Rows
Periods represent elements with the same number of electron shells. As you move across a period from left to right, the number of protons and electrons increases, leading to changes in atomic size and electronegativity. Elements within a period exhibit a diverse range of properties, transitioning from highly reactive metals to less reactive nonmetals and finally to noble gases.
Groups: Shared Properties Down the Columns
In contrast to periods, groups or families are columns that contain elements sharing similar chemical properties. This similarity stems from the fact that elements within the same group have the same number of valence electrons – the electrons in the outermost shell. These valence electrons are primarily responsible for an element's chemical behavior, determining its reactivity and the types of bonds it can form.
The Significance of Groupings: Predicting Chemical Behavior
The grouping of elements allows chemists to predict their behavior. Knowing an element's group provides crucial insights into its reactivity, bonding tendencies, and the types of compounds it will form. This predictive power is invaluable in various fields, including materials science, drug discovery, and industrial chemistry.
Key Group Characteristics
Each group exhibits unique characteristics. Let's explore some of the prominent groups and their key properties:
1. Alkali Metals (Group 1): These highly reactive metals have one valence electron, readily losing it to form +1 ions. They are soft, silvery-white metals, highly reactive with water, and readily form ionic compounds. Examples include lithium (Li), sodium (Na), and potassium (K).
2. Alkaline Earth Metals (Group 2): Possessing two valence electrons, these metals are also reactive but less so than alkali metals. They tend to form +2 ions and are generally harder and denser than alkali metals. Examples include beryllium (Be), magnesium (Mg), and calcium (Ca).
3. Transition Metals (Groups 3-12): This large block of elements exhibits a wide range of properties. They are generally good conductors of heat and electricity, often form colored compounds, and can exist in multiple oxidation states (meaning they can lose different numbers of electrons to form ions). Iron (Fe), copper (Cu), and gold (Au) are examples. The transition metals play a vital role in various biological processes and industrial applications.
4. Boron Family (Group 13): This group shows a transition from metallic to nonmetallic behavior as you move down the group. Boron (B) is a metalloid, while aluminum (Al), gallium (Ga), and indium (In) are metals. They typically form +3 ions.
5. Carbon Family (Group 14): This group exhibits a wide range of properties, including metallic, metalloid, and nonmetallic characteristics. Carbon (C) forms the basis of organic chemistry, silicon (Si) is crucial in semiconductor technology, and tin (Sn) and lead (Pb) are well-known metals.
6. Nitrogen Family (Group 15): This group showcases a similar trend to the carbon family. Nitrogen (N) is a nonmetal essential for life, while phosphorus (P), arsenic (As), and antimony (Sb) exhibit increasing metallic character down the group. They often form -3 ions.
7. Chalcogens (Group 16): This group contains oxygen (O), sulfur (S), selenium (Se), and tellurium (Te). Oxygen is essential for respiration, while sulfur plays a crucial role in various industrial processes. They often form -2 ions.
8. Halogens (Group 17): These highly reactive nonmetals have seven valence electrons. They readily gain one electron to form -1 ions, forming ionic compounds with metals. Fluorine (F), chlorine (Cl), bromine (Br), and iodine (I) are examples of halogens.
9. Noble Gases (Group 18): These inert gases have a full valence shell (eight electrons, except helium with two), making them exceptionally unreactive. Their stability arises from their complete electron shells. Helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn) are noble gases, often used in lighting and other applications.
Beyond the Main Groups: The f-Block Elements
The periodic table also includes the f-block elements, commonly known as the lanthanides and actinides. These elements are placed separately at the bottom of the table to maintain the table's overall structure. They have unique electronic configurations and properties, playing crucial roles in various technological applications.
The Importance of Understanding Groups in Chemistry
Understanding the organization of the periodic table, particularly the characteristics of groups, is paramount in chemistry. It provides a framework for:
- Predicting chemical reactions: Knowing the group of an element allows you to anticipate its reactivity and the types of compounds it will form.
- Designing new materials: The properties of elements in specific groups are crucial for designing materials with desired characteristics.
- Understanding biological processes: Many elements in specific groups play vital roles in biological systems.
- Developing new technologies: The unique properties of elements in particular groups drive innovation across various industries.
Applications and Relevance in Various Fields
The grouping of elements is not merely an academic exercise; it has profound implications across various disciplines:
1. Materials Science: Understanding the properties of elements in different groups is essential for designing advanced materials with specific characteristics, such as strength, conductivity, or reactivity. For example, the transition metals are crucial in the development of alloys with unique properties.
2. Pharmaceutical Industry: The periodic table provides a crucial framework for designing new drugs. Understanding the chemical properties of elements allows researchers to tailor the properties of drug molecules for improved effectiveness and reduced side effects.
3. Environmental Chemistry: The environmental impact of various elements is closely linked to their chemical properties. Understanding the behavior of elements within groups is essential for managing pollution and protecting ecosystems.
4. Agricultural Science: Many elements are crucial for plant growth, and their availability impacts crop yields. Understanding the properties of elements in groups like alkali metals and alkaline earth metals is essential for optimizing fertilizer composition.
5. Nanotechnology: The unique properties of certain elements in specific groups are exploited in the field of nanotechnology to design and create novel materials and devices with enhanced properties.
Conclusion: The Periodic Table – A Powerful Tool for Understanding Chemistry
The vertical columns on the periodic table, called groups or families, represent elements with similar outer electron shell configurations, leading to shared chemical properties. This organization is not merely a classification system; it's a powerful tool that enables chemists to predict chemical behavior, design new materials, and understand fundamental processes in various fields. From the highly reactive alkali metals to the inert noble gases, each group presents a unique story, showcasing the beauty and complexity of the periodic system. Mastering the concepts of groups and periods is fundamental to success in chemistry and its diverse applications. By understanding the trends and patterns within these columns, we unlock a deeper understanding of the matter that makes up our universe.
Latest Posts
Latest Posts
-
What Is 0 885 To The Nearest Tenth
Mar 15, 2025
-
What Is The Outermost Layer Of The Earth
Mar 15, 2025
-
Cross Sectional Area Of A Cylinder
Mar 15, 2025
-
What Is The Unit Of Current
Mar 15, 2025
-
5 Letter Word Ending With On
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Vertical Columns On The Periodic Table Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
