The Three Major Components In A Nucleotide Are
Juapaving
Mar 14, 2025 · 7 min read
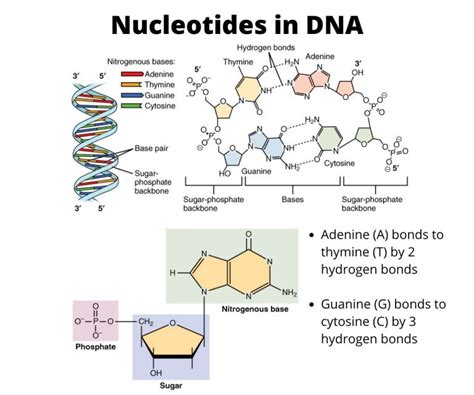
Table of Contents
The Three Major Components in a Nucleotide: A Deep Dive into the Building Blocks of Life
Nucleotides are the fundamental building blocks of nucleic acids, the crucial molecules that store and transmit genetic information in all living organisms. Understanding the three major components of a nucleotide – a nitrogenous base, a pentose sugar, and a phosphate group – is essential to grasping the intricacies of DNA, RNA, and their roles in cellular processes. This article delves deep into the structure and function of each component, highlighting their interconnectedness and the vital role they play in the molecular machinery of life.
1. The Nitrogenous Base: The Information Carrier
The nitrogenous base is the information-carrying component of a nucleotide. These bases are aromatic, heterocyclic organic molecules containing nitrogen atoms within their ring structures. They are broadly classified into two categories based on their chemical structure: purines and pyrimidines.
1.1 Purines: Adenine and Guanine
Purines are characterized by a six-membered ring fused to a five-membered ring. In nucleotides, the two principal purine bases are adenine (A) and guanine (G).
-
Adenine (A): Adenine is a crucial component of both DNA and RNA. It pairs with thymine (T) in DNA and uracil (U) in RNA through hydrogen bonds, forming the foundation of the genetic code. Adenine also plays a critical role in energy metabolism as a component of adenosine triphosphate (ATP), the primary energy currency of cells.
-
Guanine (G): Guanine, like adenine, is a component of both DNA and RNA. It pairs with cytosine (C) through hydrogen bonds, contributing to the stability and structure of the double helix in DNA and the diverse secondary structures of RNA.
1.2 Pyrimidines: Cytosine, Thymine, and Uracil
Pyrimidines possess a single six-membered ring structure. The three major pyrimidines found in nucleic acids are cytosine (C), thymine (T), and uracil (U).
-
Cytosine (C): Cytosine is found in both DNA and RNA, pairing with guanine (G) through hydrogen bonds. Its presence is essential for maintaining the integrity of the genetic code.
-
Thymine (T): Thymine is a pyrimidine base found exclusively in DNA. It pairs specifically with adenine (A), forming two hydrogen bonds and contributing to the stability of the DNA double helix.
-
Uracil (U): Uracil replaces thymine in RNA. It pairs with adenine (A) through two hydrogen bonds, playing a crucial role in RNA structure and function. The presence of uracil instead of thymine is one of the key differences between DNA and RNA.
2. The Pentose Sugar: The Structural Backbone
The second major component of a nucleotide is a pentose sugar, a five-carbon sugar molecule. The specific pentose sugar differs between DNA and RNA, significantly influencing their structures and properties.
2.1 Deoxyribose in DNA
In DNA, the pentose sugar is 2-deoxyribose. The "deoxy" prefix indicates the absence of a hydroxyl (-OH) group at the 2' carbon atom. This structural difference contributes to the greater stability of DNA compared to RNA. The absence of the 2'-OH group makes DNA less susceptible to hydrolysis (breakdown by water) than RNA. The deoxyribose sugar forms the backbone of the DNA molecule, linking the phosphate groups and nitrogenous bases.
2.2 Ribose in RNA
In RNA, the pentose sugar is ribose. Ribose differs from deoxyribose by possessing a hydroxyl (-OH) group at the 2' carbon atom. This hydroxyl group makes RNA less stable than DNA, more susceptible to hydrolysis, and contributes to its more flexible and diverse three-dimensional structures. The ribose sugar forms the backbone of the RNA molecule, similarly connecting the phosphate groups and nitrogenous bases.
3. The Phosphate Group: The Energy Source and Linkage
The phosphate group is the third crucial component of a nucleotide. It's a negatively charged molecule (PO43-) that provides several essential functions.
3.1 Linkage and Backbone Formation
The phosphate group connects the 3' carbon of one pentose sugar to the 5' carbon of the next, forming the phosphodiester bond that links nucleotides together to create the polynucleotide chain – the backbone of DNA and RNA. This linkage forms a directional backbone, with a 5' end (containing a free phosphate group) and a 3' end (containing a free hydroxyl group). This directionality is crucial for many enzymatic processes involving nucleic acids, such as DNA replication and transcription.
3.2 Energy Transfer
The phosphate groups also play a central role in energy transfer within the cell. ATP (adenosine triphosphate), for instance, is a nucleotide containing adenine, ribose, and three phosphate groups. The high-energy bonds between these phosphate groups provide the energy needed for numerous cellular processes, including muscle contraction, protein synthesis, and active transport. The hydrolysis of ATP to ADP (adenosine diphosphate) releases this energy, driving cellular work. Other nucleoside triphosphates, such as GTP (guanosine triphosphate), CTP (cytidine triphosphate), and UTP (uridine triphosphate), also participate in energy transfer and metabolic pathways.
3.3 Structural Stability
The negatively charged phosphate groups contribute significantly to the overall negative charge of DNA and RNA molecules. This negative charge influences the interactions of nucleic acids with other molecules, including proteins and ions, and affects their three-dimensional structures. The electrostatic repulsion between the negatively charged phosphate groups helps to maintain the double helix structure of DNA, and also plays a role in determining the tertiary structures of RNA.
The Interplay of Nucleotide Components: A Synergistic Relationship
The three components of a nucleotide – the nitrogenous base, the pentose sugar, and the phosphate group – are intricately interconnected. Their unique properties and interactions are crucial for the functions of DNA and RNA. The nitrogenous base carries the genetic information, the pentose sugar provides the structural backbone, and the phosphate group links the nucleotides and contributes to the stability and function of the nucleic acid molecule.
The nitrogenous base determines the sequence of genetic information. The specific order of these bases dictates the genetic code, guiding protein synthesis and regulating cellular processes. This sequence is crucial for heredity and the transmission of traits from one generation to the next.
The pentose sugar contributes to the overall structure and stability of the molecule. The difference between ribose and deoxyribose significantly influences the stability and function of RNA and DNA. The presence of the 2'-OH group in ribose makes RNA more susceptible to hydrolysis and enables it to adopt a wider range of secondary structures, crucial for its diverse roles in gene expression and regulation.
The phosphate group links the nucleotides together forming the backbone. The phosphodiester bond that unites nucleotides is critical for the structural integrity and stability of DNA and RNA. The negative charges on the phosphate groups influence the molecule's interaction with other molecules, and its overall three-dimensional structure. The phosphate group's involvement in energy transfer is vital for cellular processes. The energy released from ATP hydrolysis powers numerous cellular functions, highlighting the multifaceted roles of this crucial component.
Nucleotide Modifications: Expanding Functionality
Beyond the basic structure, nucleotides can undergo various modifications, expanding their functional repertoire. These modifications can alter their properties, influencing the structure and function of DNA and RNA. Examples include methylation of cytosine bases in DNA, influencing gene expression, and various modifications to RNA bases, affecting RNA structure and stability. These modifications highlight the dynamic nature of nucleotides and their crucial role in regulating cellular processes.
Conclusion: The Foundation of Life
In conclusion, the three major components of a nucleotide – the nitrogenous base, the pentose sugar, and the phosphate group – are inseparable, forming a synergistic unit fundamental to life. Their unique properties and interactions define the structure and function of DNA and RNA, the molecules responsible for storing, transmitting, and utilizing genetic information. Understanding the intricacies of nucleotide structure and function is essential for comprehending the complexity and beauty of the molecular machinery that drives life itself. Further research continues to unveil the vast array of roles nucleotides play in cellular processes, highlighting their crucial importance in understanding the fundamental mechanisms of biology. The ongoing investigation into nucleotide modifications and their effects on gene expression and cellular regulation promises further insights into the intricate workings of life.
Latest Posts
Latest Posts
-
What Is Pure Breeding In Genetics
May 09, 2025
-
Is 6 A Multiple Of 12
May 09, 2025
-
Molal Boiling Point Elevation Constant Table
May 09, 2025
-
What Is The Improper Fraction For 2 3 4
May 09, 2025
-
120 In Equals How Many Feet
May 09, 2025
Related Post
Thank you for visiting our website which covers about The Three Major Components In A Nucleotide Are . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.