The Most Reactive Group Of The Nonmetals Are The
Juapaving
Apr 06, 2025 · 6 min read
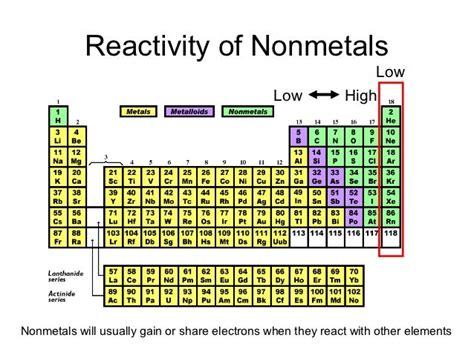
Table of Contents
The Most Reactive Group of Nonmetals: The Halogens
The periodic table organizes elements based on their properties, revealing trends and patterns in reactivity. Among the nonmetals, one group stands out for its exceptional reactivity: the halogens. This article will delve deep into the reasons behind the halogens' high reactivity, exploring their electronic configuration, chemical properties, and the fascinating reactions they undergo. We'll also examine the trends in reactivity within the halogen group itself, showcasing how these elements interact with other substances and the applications stemming from this reactivity.
Understanding the Halogens: Group 17
The halogens, located in Group 17 (VIIA) of the periodic table, consist of five elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). These elements are characterized by their high electronegativity and a strong tendency to gain an electron to achieve a stable electron configuration, mimicking the noble gases. This drive for stability is the root cause of their remarkable reactivity.
Electronic Configuration and Reactivity
The key to understanding the halogens' reactivity lies in their electronic configuration. Each halogen atom has seven valence electrons in its outermost electron shell. This means they are only one electron short of achieving a stable octet, the electron configuration of the nearest noble gas. This near-perfect stability pushes them to aggressively seek that missing electron, making them excellent oxidizing agents. They readily accept electrons from other atoms or molecules, forming negatively charged ions called halides (F⁻, Cl⁻, Br⁻, I⁻, At⁻).
Fluorine, the lightest halogen, is the most reactive of the group. Its small atomic size and high electronegativity contribute to its exceptionally strong attraction for electrons. This makes it incredibly aggressive in its reactions. Chlorine, bromine, and iodine follow in decreasing order of reactivity, with astatine, due to its radioactivity and limited availability, being less well-studied.
The Chemistry of Reactivity: Reactions of Halogens
The halogens exhibit a range of fascinating reactions, showcasing their high reactivity across various classes of compounds and elements. These reactions often involve oxidation-reduction (redox) processes, where the halogen gains electrons (reduction) and the other reactant loses electrons (oxidation).
Reactions with Metals: Formation of Halides
Halogens readily react with most metals to form ionic halides. These reactions involve the transfer of electrons from the metal to the halogen, resulting in the formation of a metal cation and a halide anion. For example, the reaction between sodium (Na) and chlorine (Cl₂) produces sodium chloride (NaCl), common table salt:
2Na(s) + Cl₂(g) → 2NaCl(s)
The strength of the metallic bond and the electronegativity difference between the metal and halogen influences the reaction rate and the stability of the resulting halide. Generally, metals with lower ionization energies react more vigorously with halogens.
Reactions with Nonmetals: Covalent Compounds
Halogens also react with nonmetals, forming covalent compounds. These reactions involve the sharing of electrons between the halogen atom and the nonmetal atom. The reactivity depends on the electronegativity difference between the reacting atoms. For instance, halogens can react with hydrogen to form hydrogen halides (HF, HCl, HBr, HI), which are highly acidic gases when dissolved in water.
H₂(g) + Cl₂(g) → 2HCl(g)
Reactions with Other Halogens: Interhalogen Compounds
Remarkably, halogens can react with each other to form interhalogen compounds. These compounds are molecules containing two or more different halogen atoms. For example, chlorine and fluorine can react to form chlorine monofluoride (ClF), chlorine trifluoride (ClF₃), and chlorine pentafluoride (ClF₅). The reactivity in these reactions is driven by the difference in electronegativity between the participating halogen atoms. Fluorine, being the most electronegative, is often the central atom in these compounds.
Displacement Reactions: Halogen Reactivity Series
The halogens demonstrate a clear trend in their reactivity, reflected in displacement reactions. A more reactive halogen can displace a less reactive halogen from its compound. For instance, chlorine can displace bromine from a bromide solution:
Cl₂(aq) + 2KBr(aq) → 2KCl(aq) + Br₂(aq)
This reactivity series, F₂ > Cl₂ > Br₂ > I₂, highlights the decreasing reactivity down the group. Fluorine, being the most reactive, can displace all other halogens. Chlorine can displace bromine and iodine, while bromine can only displace iodine.
Applications of Halogen Reactivity
The high reactivity of halogens translates into numerous applications across diverse fields:
-
Water purification: Chlorine is widely used as a disinfectant in water treatment plants, eliminating harmful bacteria and viruses. Its oxidizing power effectively kills pathogens, ensuring safe drinking water.
-
Medical applications: Halogens play a vital role in medicine. Iodine, for example, is a common antiseptic, used to sterilize wounds and prevent infections. Chlorine compounds are also used in some medications.
-
Industrial processes: Halogens find widespread use in various industrial processes. Chlorine is used in the production of PVC (polyvinyl chloride) plastics, a versatile material used in countless applications. Fluorine compounds are crucial in the manufacturing of refrigerants and Teflon.
-
Pesticides and herbicides: Some halogen-containing compounds are used as pesticides and herbicides. However, concerns about their environmental impact and persistence have led to restrictions on their use in many regions.
Trends in Halogen Reactivity: Atomic Radius and Electronegativity
The reactivity of halogens decreases down the group (F > Cl > Br > I). This trend can be attributed to two key factors:
-
Atomic Radius: As you move down the group, the atomic radius increases. This means the outermost electrons are farther from the nucleus, experiencing a weaker attraction. The weaker this attraction, the less readily the atom will gain an electron to achieve a stable octet.
-
Electronegativity: Electronegativity, the ability of an atom to attract electrons in a chemical bond, also decreases down the group. Fluorine has the highest electronegativity, meaning it strongly attracts electrons. As you go down, the electronegativity decreases, resulting in less effective electron attraction.
These factors collectively explain why fluorine is the most reactive halogen, readily accepting an electron and exhibiting vigorous reactions, whereas iodine is the least reactive, showing comparatively slower reaction rates.
Conclusion: The Reign of Reactive Halogens
The halogens, with their distinctive electronic configuration and high electronegativity, constitute the most reactive group of nonmetals. Their tendency to readily gain an electron to achieve a stable octet drives a wide array of chemical reactions, ranging from the formation of ionic halides with metals to the creation of covalent compounds with nonmetals. Their reactivity is exploited in numerous applications, from essential water purification to diverse industrial processes. However, understanding their reactivity and potential environmental impacts is crucial for responsible use and mitigating any negative consequences. The detailed study of halogens' reactivity continues to deepen our understanding of chemical bonding and offers exciting possibilities for future applications in diverse scientific and technological fields.
Latest Posts
Latest Posts
-
What Is The Longest Stage Of The Cell Cycle
Apr 08, 2025
-
Least Common Multiple Of 18 And 12
Apr 08, 2025
-
How Many Meters In A Kilogram
Apr 08, 2025
-
How Many Acres Are In One Hectare
Apr 08, 2025
-
Words That Begin With A G
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about The Most Reactive Group Of The Nonmetals Are The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.