The Most Abundant Gas In The Atmosphere Is
Juapaving
Mar 20, 2025 · 5 min read
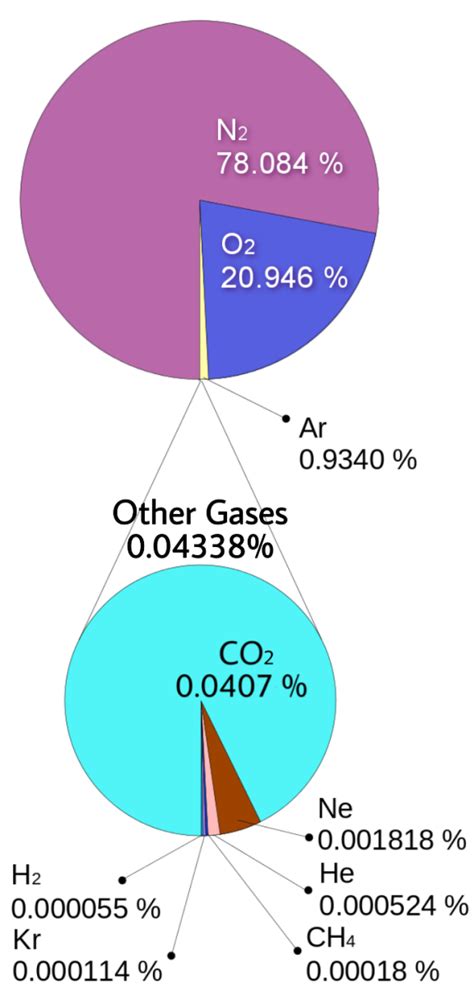
Table of Contents
The Most Abundant Gas in the Atmosphere Is: Nitrogen – A Deep Dive
The Earth's atmosphere is a dynamic and complex mixture of gases, vital for supporting life as we know it. While a multitude of gases contribute to its composition, one stands out as the most prevalent: nitrogen (N₂). This seemingly unremarkable gas plays a crucial role in numerous atmospheric processes and the overall health of our planet. This article delves deep into the significance of nitrogen, exploring its abundance, properties, and its indispensable role in the Earth's ecosystem.
Understanding Atmospheric Composition
Before focusing on nitrogen's dominance, let's briefly review the overall composition of the Earth's atmosphere. The atmosphere is a layered structure extending hundreds of kilometers from the Earth's surface. Its composition varies slightly with altitude, but the major constituents remain relatively consistent in the lower layers, which are most relevant to life.
The most abundant gases are:
- Nitrogen (N₂): Approximately 78%
- Oxygen (O₂): Approximately 21%
- Argon (Ar): Approximately 0.93%
The remaining fraction (less than 1%) consists of trace gases, including:
- Carbon dioxide (CO₂): A crucial greenhouse gas.
- Neon (Ne), Helium (He), Methane (CH₄), Krypton (Kr), Hydrogen (H₂), and Xenon (Xe): Present in minor quantities.
- Water vapor (H₂O): Highly variable depending on location and weather conditions.
Nitrogen: The Undisputed Champion
As the data clearly shows, nitrogen constitutes roughly 78% of the Earth's atmosphere, making it the most abundant gas by a considerable margin. This dominance is a key factor shaping our planet's environment and influencing various natural processes. But why is nitrogen so abundant?
The Nitrogen Cycle: A Continuous Process
The abundance of nitrogen is largely a consequence of the nitrogen cycle, a complex biogeochemical process that involves the continuous transformation of nitrogen between different forms and reservoirs. This cycle encompasses several key processes:
-
Nitrogen Fixation: Atmospheric nitrogen (N₂) is highly stable and relatively unreactive. To be utilized by living organisms, it needs to be "fixed," meaning converted into more reactive forms like ammonia (NH₃) or nitrates (NO₃⁻). This process occurs naturally through lightning strikes, which provide the energy needed to break the strong triple bond in N₂ molecules. It's also carried out by specialized microorganisms, particularly nitrogen-fixing bacteria found in soil and aquatic environments. These bacteria possess enzymes capable of converting atmospheric nitrogen into usable forms for plants.
-
Nitrification: Ammonia produced through nitrogen fixation is further oxidized by other bacteria in the soil to form nitrites (NO₂⁻) and then nitrates (NO₃⁻). These forms are readily absorbed by plants as nutrients.
-
Assimilation: Plants incorporate nitrates into their tissues, using them to synthesize essential organic compounds like amino acids and proteins. Animals then obtain nitrogen by consuming plants or other animals.
-
Ammonification: When organisms die and decompose, the nitrogen contained within their tissues is released back into the environment in the form of ammonia.
-
Denitrification: Certain bacteria convert nitrates back into atmospheric nitrogen (N₂), completing the cycle. This process occurs under anaerobic (oxygen-deficient) conditions, often in waterlogged soils or sediments.
The Inert Nature of Nitrogen Gas
Despite its abundance, the inert nature of diatomic nitrogen (N₂) gas is a critical factor. The strong triple bond between the two nitrogen atoms makes it exceptionally stable and unreactive at normal temperatures and pressures. This inertness prevents it from readily participating in many chemical reactions, explaining its persistence in the atmosphere. This stability also makes nitrogen a relatively safe component of the atmosphere, unlike some other gases that are highly reactive and potentially harmful.
The Importance of Nitrogen in Life
Although the inert nature of atmospheric nitrogen limits its direct use by most organisms, the nitrogen cycle transforms it into essential compounds crucial for life.
-
Protein Synthesis: Nitrogen is a fundamental building block of amino acids, the basic units of proteins. Proteins are vital for the structure, function, and regulation of all living organisms. From enzymes that catalyze biochemical reactions to structural components like collagen, proteins are indispensable for life.
-
Nucleic Acid Formation: Nitrogen is also a key component of nucleic acids—DNA and RNA—which carry the genetic information essential for reproduction and inheritance. Without sufficient nitrogen, life as we know it could not exist.
-
Plant Growth: Nitrogen is one of the three major macronutrients required for plant growth (along with phosphorus and potassium). Nitrogen deficiency leads to stunted growth, reduced yields, and overall poor plant health. This makes nitrogen fertilization a significant aspect of modern agriculture.
Human Impact on the Nitrogen Cycle
Human activities have significantly altered the nitrogen cycle, leading to both positive and negative consequences.
-
Haber-Bosch Process: The industrial synthesis of ammonia, known as the Haber-Bosch process, has revolutionized agriculture by enabling large-scale production of nitrogen fertilizers. This process, while increasing food production, also contributes to significant nitrogen pollution.
-
Fossil Fuel Combustion: The burning of fossil fuels releases significant amounts of nitrogen oxides (NOx) into the atmosphere, contributing to air pollution, acid rain, and the formation of ground-level ozone.
-
Deforestation and Land Use Change: Changes in land use patterns can disrupt the nitrogen cycle, affecting the balance between nitrogen fixation, nitrification, and denitrification.
Environmental Concerns Related to Nitrogen
Excess nitrogen from human activities has several detrimental environmental consequences:
-
Eutrophication: Excess nitrogen runoff from agricultural fields and other sources can cause eutrophication in aquatic ecosystems. This process leads to excessive algal growth, depleting oxygen levels and harming aquatic life.
-
Acid Rain: Nitrogen oxides contribute to acid rain, which can damage forests, lakes, and buildings.
-
Greenhouse Effect: Nitrous oxide (N₂O), a potent greenhouse gas, is released through various human activities, contributing to climate change.
Conclusion: A Gas of Profound Importance
While often overlooked due to its inert nature, nitrogen's abundance and pivotal role in the nitrogen cycle make it a gas of profound importance. Its significance extends far beyond its simple presence in the atmosphere. Understanding the nitrogen cycle and the impacts of human activities on this crucial process is essential for mitigating environmental problems and ensuring a sustainable future. Further research into optimizing nitrogen use in agriculture and mitigating nitrogen pollution remains crucial for protecting the health of our planet and ensuring food security for a growing global population. The seemingly simple gas, nitrogen, is a cornerstone of life on Earth, and its continued study and responsible management are paramount to our planet's well-being.
Latest Posts
Latest Posts
-
How To Understand A Circuit Diagram
May 09, 2025
-
What Is The Significance Of Chemical Formula
May 09, 2025
-
3 1 4 As Improper Fraction
May 09, 2025
-
What Organelles Involved In Protein Synthesis
May 09, 2025
-
How Many Feet Is 25 Centimeters
May 09, 2025
Related Post
Thank you for visiting our website which covers about The Most Abundant Gas In The Atmosphere Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.