The Mass Per Unit Volume Of A Substance Is Called
Juapaving
Mar 23, 2025 · 6 min read
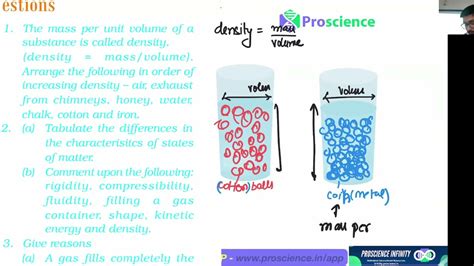
Table of Contents
- The Mass Per Unit Volume Of A Substance Is Called
- Table of Contents
- The Mass Per Unit Volume of a Substance is Called: Density – A Deep Dive
- Understanding Density: Mass and Volume in Harmony
- Units of Density
- Factors Affecting Density: Temperature and Pressure
- Temperature's Influence on Density
- Pressure's Impact on Density
- Calculating Density: Practical Examples
- Density in Different States of Matter
- Solids
- Liquids
- Gases
- Applications of Density: A Wide Spectrum
- Density and its Relationship with other Properties
- Conclusion: The Significance of Density
- Latest Posts
- Latest Posts
- Related Post
The Mass Per Unit Volume of a Substance is Called: Density – A Deep Dive
The mass per unit volume of a substance is called density. This seemingly simple concept is fundamental to physics, chemistry, and engineering, influencing everything from buoyancy and material selection to the behavior of gases and the design of aircraft. Understanding density goes beyond simply memorizing a definition; it involves grasping its implications across diverse scientific fields and everyday applications. This article delves into the intricacies of density, exploring its calculation, units, variations with temperature and pressure, and its significance in various contexts.
Understanding Density: Mass and Volume in Harmony
Density is a measure of how much mass is packed into a given volume. It's a crucial property because it tells us how tightly the atoms or molecules of a substance are arranged. A high density indicates tightly packed particles, while a low density signifies more space between particles. The formal definition is:
Density (ρ) = Mass (m) / Volume (V)
This equation tells us that density is directly proportional to mass and inversely proportional to volume. This means that if you increase the mass of a substance while keeping the volume constant, its density will increase. Conversely, if you increase the volume while keeping the mass constant, its density will decrease.
Units of Density
The standard unit of density in the International System of Units (SI) is kilograms per cubic meter (kg/m³). However, other units are commonly used depending on the context:
- grams per cubic centimeter (g/cm³): This is a particularly convenient unit for many applications, especially in chemistry, because 1 g/cm³ is numerically equal to 1000 kg/m³.
- pounds per cubic foot (lb/ft³): This unit is frequently used in engineering applications in countries using the imperial system.
- grams per milliliter (g/mL): Since 1 mL is equal to 1 cm³, this unit is equivalent to g/cm³.
The choice of unit depends on the scale and nature of the problem being addressed. For macroscopic objects like cars or buildings, kg/m³ might be appropriate. For microscopic objects or substances at a molecular level, g/cm³ or g/mL might be more practical.
Factors Affecting Density: Temperature and Pressure
Density is not always a constant property of a substance. It can be significantly influenced by two key factors: temperature and pressure.
Temperature's Influence on Density
Generally, an increase in temperature causes a decrease in density for most substances. This is because heating a substance causes its particles to move faster and spread further apart, resulting in an increase in volume while the mass remains constant. This inversely affects density according to the formula above. The exception to this rule is water, which exhibits anomalous behavior between 0°C and 4°C. In this temperature range, water's density increases as temperature increases, reaching a maximum at 4°C before decreasing as it continues to warm. This unique characteristic of water has significant implications for aquatic life and the Earth's climate.
Pressure's Impact on Density
Increased pressure generally leads to increased density. This is because higher pressure compresses the substance, reducing its volume while the mass remains unchanged. This effect is particularly pronounced in gases, which are highly compressible. Liquids and solids are less compressible, so the effect of pressure on their density is less dramatic but still measurable.
Calculating Density: Practical Examples
Calculating the density of a substance is often a straightforward process, provided you can measure its mass and volume accurately. Let's illustrate with a few examples:
Example 1: A regular solid
Imagine a rectangular block of metal with a mass of 270 grams. Its dimensions are 5 cm x 3 cm x 2 cm. To calculate the density:
- Calculate the volume: Volume = length x width x height = 5 cm x 3 cm x 2 cm = 30 cm³
- Calculate the density: Density = Mass / Volume = 270 g / 30 cm³ = 9 g/cm³
Example 2: An irregular solid
For an irregularly shaped object, you can use water displacement to determine its volume. Submerge the object in a graduated cylinder filled with water and measure the increase in water level. The difference represents the volume of the object.
Example 3: A liquid
The density of a liquid is usually determined by weighing a known volume of the liquid using a graduated cylinder or pipette.
Density in Different States of Matter
Density varies significantly across the three common states of matter: solid, liquid, and gas.
Solids
Solids generally have the highest densities because their particles are closely packed together in a fixed arrangement. However, the density of solids can vary widely depending on the substance's atomic structure and intermolecular forces.
Liquids
Liquids have intermediate densities compared to solids and gases. Their particles are closer together than in a gas but are not as rigidly arranged as in a solid. The density of a liquid is influenced by factors like temperature and the presence of dissolved substances.
Gases
Gases have the lowest densities because their particles are widely spaced and move freely. Their density is highly sensitive to changes in temperature and pressure.
Applications of Density: A Wide Spectrum
The concept of density finds extensive applications across numerous fields. Some prominent examples include:
-
Archimedes' Principle and Buoyancy: The principle of buoyancy states that an object immersed in a fluid experiences an upward buoyant force equal to the weight of the fluid displaced by the object. This is directly related to density. Objects with a density less than the fluid will float, while those with higher density will sink.
-
Material Selection in Engineering: Engineers consider density when choosing materials for various applications. Lightweight materials with high strength-to-weight ratios, such as aluminum alloys, are preferred in aerospace and automotive industries.
-
Hydrostatic Pressure: The pressure exerted by a fluid at rest is directly proportional to its density and the depth within the fluid. This is a critical concept in oceanography and hydraulics.
-
Atmospheric Science: Understanding the density variations in the Earth's atmosphere is crucial for weather forecasting and climate modeling. Density differences drive air circulation patterns.
-
Medical Imaging: Different tissues in the human body have different densities, which are exploited in medical imaging techniques like X-rays and computed tomography (CT) scans. These techniques rely on the differential absorption of X-rays by tissues of varying density.
Density and its Relationship with other Properties
Density is intricately linked with other physical properties of a substance, including:
-
Specific Gravity: This is the ratio of a substance's density to the density of a reference substance, typically water at 4°C. Specific gravity is a dimensionless quantity, making it convenient for comparisons.
-
Molar Volume: This is the volume occupied by one mole of a substance. It's inversely proportional to density.
-
Concentration: In solutions, density is related to concentration, which is the amount of solute dissolved in a given amount of solvent.
Conclusion: The Significance of Density
The mass per unit volume of a substance—its density—is far more than a simple definition. It's a fundamental property that underpins a vast array of physical phenomena and engineering applications. Understanding density, its variations with temperature and pressure, and its relationships with other properties is crucial across various scientific and technological fields. From designing aircraft to understanding weather patterns and interpreting medical images, the concept of density plays a vital role. Its importance extends far beyond the textbook definition, shaping our understanding of the world around us.
Latest Posts
Latest Posts
-
What Are A Group Of Tissues Working Together Called
Mar 25, 2025
-
What Is 6 7 As A Percent
Mar 25, 2025
-
How To Prove Two Triangles Are Similar
Mar 25, 2025
-
Which Of The Following Muscles Is Voluntary
Mar 25, 2025
-
How To Find The Experimental Probability
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about The Mass Per Unit Volume Of A Substance Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
