The Mass Per Unit Volume Of A Substance
Juapaving
Mar 13, 2025 · 6 min read
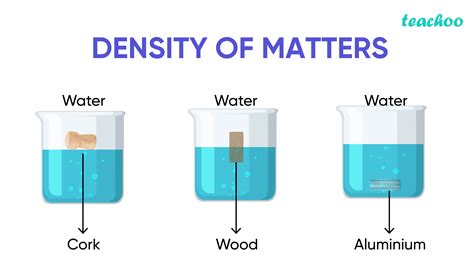
Table of Contents
The Mass Per Unit Volume of a Substance: Density Explained
Density, a fundamental concept in physics and chemistry, describes the mass per unit volume of a substance. It's a crucial property that helps us understand and characterize materials, predict their behavior, and solve a wide range of problems in various scientific fields and everyday applications. This article delves deep into the concept of density, exploring its definition, calculation, units, applications, and factors affecting it. We will also examine the relationship between density and other physical properties and discuss how density plays a vital role in various scientific and engineering disciplines.
Understanding Density: Mass Divided by Volume
At its core, density is a measure of how much mass is packed into a given volume. A substance with high density has a lot of mass crammed into a small volume, while a substance with low density has less mass spread over the same volume. The mathematical representation of density is straightforward:
Density (ρ) = Mass (m) / Volume (V)
Where:
- ρ (rho) represents density, typically expressed in units of kilograms per cubic meter (kg/m³) or grams per cubic centimeter (g/cm³).
- m represents mass, usually measured in kilograms (kg) or grams (g).
- V represents volume, typically expressed in cubic meters (m³) or cubic centimeters (cm³).
Units of Density: A Closer Look
The choice of units for density depends on the context and the system of units being used. The most common units are:
- kg/m³ (kilograms per cubic meter): This is the SI unit for density and is widely used in scientific and engineering calculations.
- g/cm³ (grams per cubic centimeter): This unit is frequently used in chemistry and materials science, especially when dealing with smaller samples.
- g/mL (grams per milliliter): This unit is equivalent to g/cm³ and is often used for liquids.
Calculating Density: Methods and Examples
Calculating the density of a substance involves determining its mass and volume. The methods used to measure mass and volume vary depending on the state of matter (solid, liquid, or gas) and the nature of the substance.
Measuring Mass: Using a Balance
Mass is typically determined using a balance, an instrument that measures the force of gravity acting on an object. Digital balances offer high precision and accuracy, while traditional beam balances provide a visual representation of mass comparison.
Measuring Volume: Techniques for Different States of Matter
Measuring volume depends on the state of matter:
- Solids: For regularly shaped solids (cubes, spheres, cylinders), the volume can be calculated using geometric formulas. For irregularly shaped solids, water displacement is a common technique. The object is submerged in a known volume of water, and the change in water level corresponds to the object's volume.
- Liquids: The volume of liquids is easily measured using graduated cylinders, beakers, or volumetric flasks.
- Gases: Measuring the volume of gases requires specialized equipment, such as gas syringes or respirometers, and often involves considering temperature and pressure.
Examples of Density Calculations
Let's illustrate density calculations with two examples:
Example 1: A solid cube
A cube of iron has a mass of 7870 g and sides of length 10 cm. Its volume is 10 cm * 10 cm * 10 cm = 1000 cm³. Therefore, its density is:
Density = Mass / Volume = 7870 g / 1000 cm³ = 7.87 g/cm³
Example 2: A liquid
A 50 mL sample of ethanol has a mass of 39.5 g. Its density is:
Density = Mass / Volume = 39.5 g / 50 mL = 0.79 g/mL = 0.79 g/cm³
Factors Affecting Density: Temperature, Pressure, and Composition
Several factors can influence the density of a substance:
- Temperature: Generally, as temperature increases, the volume of a substance increases (due to thermal expansion), while the mass remains relatively constant. This leads to a decrease in density. However, this relationship isn't universal; water exhibits an anomalous behavior with its maximum density at 4°C.
- Pressure: Pressure primarily affects the density of gases. Increasing pressure compresses the gas, reducing its volume and increasing its density. The effect of pressure on the density of liquids and solids is generally negligible.
- Composition: The density of a mixture or solution depends on the densities and proportions of its components. For instance, adding salt to water increases the density of the solution. The chemical composition of a substance fundamentally determines its density. Different isotopes of the same element will also exhibit slightly different densities due to variations in their mass.
Applications of Density: Across Various Disciplines
Density is a critical property with wide-ranging applications:
- Material Identification: Density is a characteristic property that helps identify unknown substances. Comparing the density of an unknown sample to known values can aid in material identification.
- Industrial Processes: Density measurements are vital in various industrial processes, including quality control, process optimization, and mixture preparation. Examples include monitoring the concentration of solutions, ensuring consistent product quality, and controlling fluid flow in pipelines.
- Geology and Geophysics: Density variations within the Earth's layers provide insights into its structure and composition. Geophysicists utilize density measurements to understand subsurface structures and locate mineral deposits.
- Oceanography: Oceanographers use density measurements to study ocean currents, water mixing, and the distribution of marine life. Density gradients play a crucial role in determining water column stratification.
- Medicine: Density measurements are used in medical imaging techniques, such as bone densitometry (to measure bone density) and blood tests (to determine blood components).
- Aerospace Engineering: Density is crucial in aerospace engineering for designing aircraft and spacecraft. Air density affects lift and drag forces, influencing aircraft performance. Density variations in the atmosphere also influence spacecraft trajectories and atmospheric re-entry.
Relative Density and Specific Gravity: Related Concepts
Closely related to density are relative density and specific gravity:
- Relative Density: This is the ratio of the density of a substance to the density of a reference substance (usually water at 4°C). It's a dimensionless quantity, indicating how many times denser or lighter a substance is compared to the reference.
- Specific Gravity: Specific gravity is essentially the same as relative density, but the term is often used in engineering and industry. It's also a dimensionless quantity and expresses the density ratio compared to water.
Density and Buoyancy: Archimedes' Principle
Density plays a key role in understanding buoyancy, as described by Archimedes' principle: An object immersed in a fluid experiences an upward buoyant force equal to the weight of the fluid displaced by the object. If the object's density is less than the fluid's density, the buoyant force will be greater than the object's weight, causing it to float. Conversely, if the object's density is greater than the fluid's density, it will sink.
Conclusion: The Importance of Density in Science and Engineering
Density, the mass per unit volume of a substance, is a fundamental physical property with significant implications across diverse scientific and engineering disciplines. Understanding its calculation, units, influencing factors, and applications is crucial for solving problems in various fields, from material science and industrial processes to geology and medicine. Its importance lies in its ability to characterize substances, predict their behavior, and provide insights into the composition and structure of matter. The concept of density, therefore, remains a cornerstone of scientific understanding and technological advancement.
Latest Posts
Latest Posts
-
Which Of The Following Is A Multiple Of 5
Mar 13, 2025
-
Force Of Gravitation Between Earth And Sun
Mar 13, 2025
-
2 Out Of 5 In Percentage
Mar 13, 2025
-
Which Of The Following Religions Is Not Monotheistic
Mar 13, 2025
-
Lowest Common Multiple Of 2 And 6
Mar 13, 2025
Related Post
Thank you for visiting our website which covers about The Mass Per Unit Volume Of A Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
