The Hydrolysis Of Atp Yields Adp Phosphate Ion And
Juapaving
Mar 22, 2025 · 6 min read
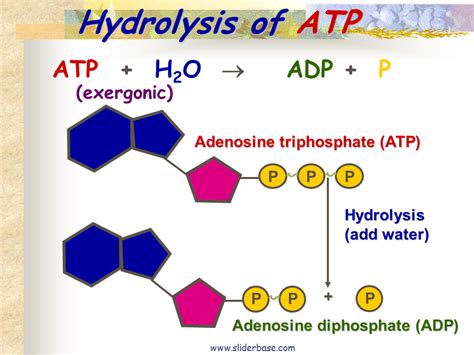
Table of Contents
The Hydrolysis of ATP: Yielding ADP, Phosphate Ions, and Cellular Energy
The hydrolysis of adenosine triphosphate (ATP) to adenosine diphosphate (ADP) and inorganic phosphate (Pi) is arguably the most fundamental reaction in all living organisms. It's the primary energy currency of cells, powering countless biological processes from muscle contraction and nerve impulse transmission to protein synthesis and DNA replication. Understanding the intricacies of ATP hydrolysis is key to comprehending the very essence of life itself. This article will delve deep into this crucial process, exploring its mechanism, energetics, and vital roles within cellular machinery.
Understanding the Structure of ATP
Before delving into the hydrolysis process, it's crucial to understand the structure of ATP itself. ATP is a nucleoside triphosphate, consisting of:
- Adenine: A nitrogenous base, a crucial component of DNA and RNA.
- Ribose: A five-carbon sugar, forming the backbone of the molecule.
- Three Phosphate Groups: These are linked together by high-energy phosphoanhydride bonds. These bonds are the key to ATP's energy-storage capabilities.
The phosphate groups are denoted as α, β, and γ, with the γ-phosphate being the terminal phosphate group, most readily cleaved during hydrolysis. The bonds between these phosphate groups are inherently unstable due to the negative charges repelling each other. This instability is precisely what makes the release of energy during hydrolysis so favorable.
The Hydrolysis Reaction: A Detailed Look
The hydrolysis of ATP can be represented by the following equation:
ATP + H₂O ⇌ ADP + Pi + Energy
This seemingly simple equation hides a complex series of events at the molecular level. The reaction involves the breaking of a phosphoanhydride bond between the β and γ phosphate groups, with a water molecule participating in the cleavage. The oxygen atom from the water molecule attacks the phosphorus atom of the γ-phosphate, leading to the formation of a new bond and the release of the γ-phosphate as inorganic phosphate (Pi).
The process isn't spontaneous; it's catalyzed by enzymes called ATPases. These enzymes significantly lower the activation energy required for the reaction to proceed, thereby accelerating the hydrolysis rate. Different ATPases exhibit varying degrees of specificity, catalyzing the hydrolysis of ATP in specific cellular contexts.
The Energetics of ATP Hydrolysis: Why is it So Important?
The hydrolysis of ATP is an exergonic reaction, meaning it releases energy. This energy release is significant, typically around -30.5 kJ/mol under standard conditions. This energy isn't released as heat; instead, it's harnessed to drive endergonic (energy-requiring) reactions within the cell. This coupling of exergonic and endergonic reactions is a central principle in cellular bioenergetics.
Several factors contribute to the high energy yield of ATP hydrolysis:
- Electrostatic Repulsion: The negative charges on the adjacent phosphate groups create significant electrostatic repulsion. Breaking this repulsion releases energy.
- Resonance Stabilization: The products of hydrolysis, ADP and Pi, are more resonance-stabilized than ATP. This increased stability contributes to the overall energy release.
- Hydration: The products of hydrolysis are more readily hydrated than ATP, contributing to the overall negative free energy change.
This energy release is not directly utilized by cellular machinery; it's transferred through various mechanisms, often involving conformational changes in proteins or the creation of high-energy intermediates.
Cellular Roles of ATP Hydrolysis: Powering Life's Processes
ATP hydrolysis fuels an incredibly diverse array of cellular processes, making it the fundamental energy currency of life. Some key examples include:
1. Muscle Contraction:
Muscle contraction relies heavily on ATP hydrolysis. Myosin, a motor protein, interacts with actin filaments, utilizing ATP hydrolysis to generate the force needed for muscle shortening and relaxation. The hydrolysis of ATP provides the energy for the conformational changes in myosin that drive this process.
2. Active Transport:
Many transport proteins within cell membranes utilize ATP hydrolysis to move molecules against their concentration gradients. This process, known as active transport, is essential for maintaining cellular homeostasis, transporting essential nutrients, and removing waste products. Examples include the sodium-potassium pump and various other ion pumps.
3. Nerve Impulse Transmission:
The transmission of nerve impulses depends on the rapid changes in membrane potential. This process involves the opening and closing of ion channels, many of which are directly or indirectly regulated by ATP hydrolysis. The energy from ATP hydrolysis is essential for restoring the resting membrane potential after an impulse has been transmitted.
4. Protein Synthesis:
Protein synthesis, the process of building proteins from amino acids, is an energy-intensive process requiring numerous ATP molecules per peptide bond formed. ATP hydrolysis is used to drive the various steps involved, including amino acid activation, ribosome translocation, and peptide bond formation.
5. DNA Replication and Repair:
DNA replication and repair are also energy-dependent processes. ATP hydrolysis provides the energy for DNA polymerase, the enzyme responsible for synthesizing new DNA strands. It also fuels the various other enzymes involved in these crucial cellular processes.
6. Cellular Signaling:
ATP itself acts as a signaling molecule in many cellular processes, and its hydrolysis is often involved in the activation or inactivation of signaling pathways. ATP hydrolysis can trigger conformational changes in proteins involved in signal transduction, influencing cellular responses to external stimuli.
Regulation of ATP Hydrolysis: Maintaining Cellular Energy Balance
The rate of ATP hydrolysis is tightly regulated to maintain cellular energy balance. This regulation is achieved through several mechanisms, including:
- Enzyme Regulation: The activity of ATPases is regulated by various factors, including allosteric modulators, covalent modifications (e.g., phosphorylation), and changes in cellular environment. This allows cells to adjust ATP hydrolysis rates based on their energy demands.
- Substrate Availability: The concentration of ATP itself influences the rate of hydrolysis. High ATP levels can inhibit ATPase activity, while low ATP levels stimulate it. This negative feedback mechanism helps maintain a stable ATP level.
- Metabolic Pathways: The rates of ATP production and consumption are intricately linked through metabolic pathways. For instance, glycolysis, the citric acid cycle, and oxidative phosphorylation regulate ATP production to meet cellular demands.
ATP Hydrolysis and Disease: The Consequences of Dysfunction
Dysfunctions in ATP hydrolysis can lead to various pathological conditions. Mutations in ATPases or defects in their regulation can have significant implications for cellular function. For example, defects in mitochondrial ATPases can lead to mitochondrial myopathies, characterized by muscle weakness and fatigue. Disruptions in ion pumps due to ATP hydrolysis impairment can contribute to various cardiovascular and neurological disorders.
Conclusion: The Universal Importance of ATP Hydrolysis
The hydrolysis of ATP to ADP and inorganic phosphate is not merely a chemical reaction; it's the engine of life. It's a fundamental process that underpins countless cellular activities, providing the energy necessary for cells to perform their essential functions. Understanding the intricacies of ATP hydrolysis is crucial for comprehending the mechanisms of life, from the molecular level to the organismal level, and for developing strategies to address diseases associated with disruptions in this crucial process. Continued research in this area remains essential for advancing our understanding of cellular bioenergetics and for developing novel therapeutic approaches for various diseases. The energy stored in the seemingly simple molecule, ATP, and its release through hydrolysis, continues to fascinate and inspire researchers across numerous biological disciplines.
Latest Posts
Latest Posts
-
Which Is Not A Property Of Living Being
Mar 22, 2025
-
In Which Two Hemispheres Is Australia Located
Mar 22, 2025
-
A Nucleotide Is Made Of Three Parts A
Mar 22, 2025
-
Energy Captured In Photosynthesis Comes From The
Mar 22, 2025
-
Square Root Of 216 In Simplest Radical Form
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about The Hydrolysis Of Atp Yields Adp Phosphate Ion And . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
