The Horizontal Rows On The Periodic Table
Juapaving
Mar 14, 2025 · 7 min read
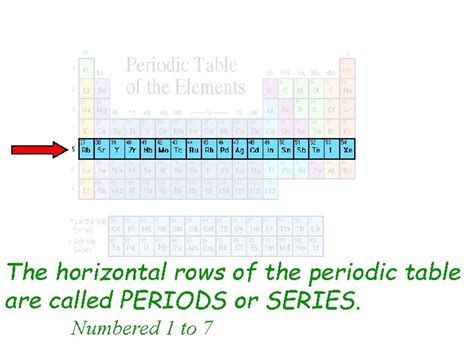
Table of Contents
Understanding the Horizontal Rows: Periods of the Periodic Table
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. While the columns (groups) represent elements with similar chemical behavior, the horizontal rows, known as periods, reveal a different yet equally crucial aspect: the gradual filling of electron shells. Understanding periods is key to grasping the trends in atomic size, ionization energy, electronegativity, and other fundamental properties. This comprehensive guide will delve into the intricacies of periods, exploring their significance and the underlying principles that govern the properties of elements within each row.
What are Periods in the Periodic Table?
Periods represent the horizontal rows in the periodic table. Each period corresponds to a principal energy level (or shell) in an atom. As we move across a period from left to right, we add one proton and one electron to the atom, systematically filling the electron orbitals within that specific energy level. This progressive filling dictates the changing properties we observe across a period. The number of elements in each period is determined by the maximum number of electrons that can occupy the orbitals within a given energy level. This number is directly related to the quantum mechanical principles governing electron configuration.
The Significance of Electron Shells and Subshells
The arrangement of electrons within an atom isn't random; it follows strict rules governed by quantum mechanics. Electrons reside in shells, and within each shell, they occupy subshells (s, p, d, and f). Each subshell can accommodate a specific number of electrons:
- s subshell: holds a maximum of 2 electrons
- p subshell: holds a maximum of 6 electrons
- d subshell: holds a maximum of 10 electrons
- f subshell: holds a maximum of 14 electrons
The filling of these subshells dictates the length and characteristics of each period. For instance, the first period is short because it only involves filling the 1s subshell (2 electrons). The second and third periods are longer because they involve filling both the s and p subshells (8 electrons total). The longer periods reflect the filling of d and f subshells, which significantly increases the number of elements.
Exploring the Periods in Detail
Let's examine each period individually, highlighting the key trends in their properties:
Period 1: The Shortest Row
Period 1 contains only two elements: hydrogen (H) and helium (He). This is the shortest period because it only involves filling the 1s subshell. Hydrogen, with one electron, is highly reactive and exists as a diatomic gas (H₂). Helium, with a full 1s subshell, is an inert noble gas, exceptionally unreactive due to its stable electron configuration.
Period 2: The Alkali Metals and Halogens Emerge
Period 2 has eight elements, starting with lithium (Li), an alkali metal, and ending with neon (Ne), a noble gas. This period introduces the 2s and 2p subshells. As we move across the period, the atomic radius decreases, ionization energy increases, and electronegativity generally increases (with some exceptions). We see the emergence of distinct chemical families: the alkali metals (Li, Be), alkaline earth metals (Na, Mg), and halogens (F, Cl). The properties of the elements in this period begin to demonstrate the periodic trends effectively.
Period 3: Similarities and Differences
Period 3, like Period 2, contains eight elements and mirrors many of the trends observed in Period 2. It begins with sodium (Na), an alkali metal, and ends with argon (Ar), a noble gas. The increased number of protons and electrons results in a larger atomic size and slightly lower ionization energies compared to their Period 2 counterparts. However, the overall trends of increasing ionization energy and electronegativity across the period remain consistent. The chemical similarities between elements in Period 3 and their Period 2 counterparts showcase the periodic nature of the table.
Period 4: Transition Metals Appear
Period 4 is longer (18 elements) due to the introduction of the 3d subshell, which is filled after the 4s subshell. This period marks the beginning of the transition metals, a group of elements characterized by their partially filled d orbitals. Transition metals exhibit variable oxidation states, meaning they can lose different numbers of electrons to form ions. This characteristic accounts for their diverse and often colorful compounds. The inclusion of transition metals introduces complexity to the periodic trends. While ionization energy and electronegativity generally increase across the period, the trends aren't as straightforward as in previous periods.
Period 5: Expanding on the Trends
Period 5, much like Period 4, also contains 18 elements due to the filling of the 4d subshell. It features more transition metals and continues to illustrate the periodic trends established in earlier periods. The increase in atomic size and the slight decrease in ionization energy compared to Period 4 are again observable. However, the effects of shielding and other subtle quantum effects become more prominent, influencing the precise values of these properties.
Period 6: Lanthanides and the Complexity of f-orbitals
Period 6, the longest period with 32 elements, contains the lanthanides (rare earth elements). The filling of the 4f subshell results in the lanthanide series, often placed separately at the bottom of the periodic table for convenience. The elements in this period display complex interactions between electrons in the 4f, 5d, and 6s orbitals, resulting in less pronounced trends in properties compared to earlier periods. The chemical similarities among lanthanides are high due to the shielding effect of the f-orbitals.
Period 7: Actinides and the End of the Known Elements
Period 7, also with 32 elements, includes the actinides, another series of elements with a partially filled 5f subshell, often placed separately at the bottom of the periodic table. The actinides are all radioactive, and most are synthetically produced. The trends in this period are even less pronounced than in Period 6 due to the complex electron interactions. Period 7 currently represents the end of the known elements in the periodic table.
Periodic Trends Across Periods
The filling of electron shells within periods leads to predictable trends in various atomic properties:
Atomic Radius:
Atomic radius generally decreases across a period. As we add protons, the increased positive charge pulls the electrons closer to the nucleus, resulting in a smaller atom.
Ionization Energy:
Ionization energy, the energy required to remove an electron from an atom, generally increases across a period. The stronger attraction between the nucleus and electrons makes it harder to remove an electron.
Electronegativity:
Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally increases across a period. Atoms with a stronger pull on electrons are more electronegative.
Metallic Character:
Metallic character generally decreases across a period. Elements on the left side of the periodic table tend to be more metallic, while those on the right are more non-metallic. This trend is closely linked to ionization energy and electronegativity.
The Importance of Understanding Periods
A thorough understanding of periods is crucial for several reasons:
- Predicting Properties: Knowing an element's period allows us to predict its general properties based on established periodic trends.
- Chemical Reactivity: The position of an element within a period helps predict its reactivity and the types of chemical bonds it will form.
- Understanding Chemical Reactions: Periods help explain why certain elements react in specific ways and how their chemical behavior is related to their electron configuration.
- Material Science: Understanding periods is fundamental to materials science, allowing us to design and synthesize materials with specific properties.
Conclusion
The horizontal rows, or periods, of the periodic table provide a vital framework for understanding the relationships between elements. The systematic filling of electron shells within each period leads to predictable trends in atomic properties. While the trends are generally consistent, complexities arise in later periods due to the filling of d and f orbitals. Mastering the concepts of periods is essential for any aspiring chemist, providing a powerful tool for predicting and understanding the behavior of elements and their compounds. Further exploration into the intricacies of quantum mechanics provides even deeper insights into these fascinating periodic trends.
Latest Posts
Latest Posts
-
What Is 1 Billion X 1 Trillion
May 09, 2025
-
Vinegar Is An Acid Or Base
May 09, 2025
-
What Is 3 Out Of 4 As A Percentage
May 09, 2025
-
Why Is The Chromosome Number Reduced By Half During Meiosis
May 09, 2025
-
How To Find Particular Solution Differential Equations
May 09, 2025
Related Post
Thank you for visiting our website which covers about The Horizontal Rows On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.