The Final Electron Acceptor Of Aerobic Cellular Respiration Is _____.
Juapaving
Mar 09, 2025 · 6 min read
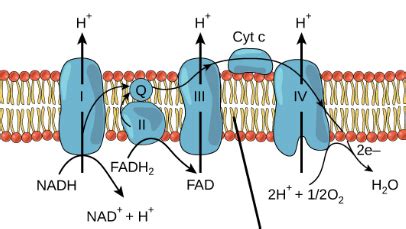
Table of Contents
The Final Electron Acceptor of Aerobic Cellular Respiration is Oxygen: A Deep Dive into Oxidative Phosphorylation
The final electron acceptor of aerobic cellular respiration is oxygen (O₂). This seemingly simple statement underpins one of the most crucial processes in biology, responsible for the energy production that fuels nearly all eukaryotic life and many prokaryotic organisms. Understanding this process, oxidative phosphorylation, is key to grasping the intricacies of cellular respiration and its importance in maintaining life. This article will delve deep into the mechanism of oxidative phosphorylation, exploring the role of oxygen, the electron transport chain (ETC), and the resulting ATP synthesis. We'll also touch upon the consequences of alternative electron acceptors and the broader implications for various life forms.
Understanding Cellular Respiration: A Recap
Before diving into the role of oxygen, let's briefly review the overall process of cellular respiration. Cellular respiration is a series of metabolic reactions that break down glucose and other nutrient molecules to generate ATP (adenosine triphosphate), the cell's primary energy currency. This process can be broadly divided into four stages:
-
Glycolysis: The initial breakdown of glucose in the cytoplasm, yielding pyruvate, a small amount of ATP, and NADH (nicotinamide adenine dinucleotide, a crucial electron carrier).
-
Pyruvate Oxidation: Pyruvate is transported into the mitochondria, where it's converted into acetyl-CoA, releasing carbon dioxide and generating more NADH.
-
Krebs Cycle (Citric Acid Cycle): Acetyl-CoA enters the Krebs cycle, a series of reactions that further oxidize the carbon atoms, releasing more carbon dioxide and generating ATP, NADH, and FADH₂ (flavin adenine dinucleotide, another electron carrier).
-
Oxidative Phosphorylation: This is where the magic happens. The NADH and FADH₂ generated in the previous stages donate their high-energy electrons to the electron transport chain, ultimately leading to the production of a significant amount of ATP. This is the stage where oxygen plays its vital role as the final electron acceptor.
The Electron Transport Chain (ETC): A Cascade of Redox Reactions
The electron transport chain is a series of protein complexes embedded in the inner mitochondrial membrane. These complexes facilitate a series of redox reactions, where electrons are passed from one molecule to another, gradually decreasing their energy level. This electron flow is coupled with the pumping of protons (H⁺ ions) across the inner mitochondrial membrane, creating a proton gradient.
This gradient represents a form of stored energy, similar to water stored behind a dam. The potential energy stored in this proton gradient is harnessed by ATP synthase, a remarkable enzyme complex that utilizes the flow of protons back across the membrane to drive the synthesis of ATP from ADP (adenosine diphosphate) and inorganic phosphate (Pi). This process is known as chemiosmosis.
The ETC comprises four major protein complexes (Complex I-IV), each with its specific role in electron transfer and proton pumping. Electrons from NADH enter the chain at Complex I, while electrons from FADH₂ enter at Complex II. As electrons move down the chain, they pass through a series of increasingly electronegative molecules, culminating in the reduction of oxygen to water.
The Crucial Role of Oxygen: The Terminal Electron Acceptor
Oxygen's role as the final electron acceptor is absolutely crucial. Without it, the electron transport chain would come to a standstill. The electrons, unable to be passed on to oxygen, would accumulate in the chain, effectively halting the flow of electrons and preventing the generation of the proton gradient necessary for ATP synthesis. This would dramatically reduce ATP production, severely impacting cellular function and ultimately leading to cell death.
The reaction where oxygen accepts electrons and protons is:
½ O₂ + 2e⁻ + 2H⁺ → H₂O
This reaction is highly exergonic, meaning it releases a significant amount of energy, which is harnessed to drive the proton pumping. The formation of water is a thermodynamically favorable process, ensuring the continuous flow of electrons through the ETC.
Alternative Electron Acceptors: Anaerobic Respiration
While oxygen is the most efficient final electron acceptor, some organisms can survive under anaerobic conditions (without oxygen) by utilizing alternative electron acceptors. This process is known as anaerobic respiration. Examples of alternative electron acceptors include:
- Nitrate (NO₃⁻): Used by certain bacteria to produce nitrite (NO₂⁻) or nitrogen gas (N₂).
- Sulfate (SO₄²⁻): Used by sulfate-reducing bacteria to produce hydrogen sulfide (H₂S).
- Carbon dioxide (CO₂): Used by methanogens to produce methane (CH₄).
These alternative electron acceptors have lower reduction potentials than oxygen, meaning they are less effective at accepting electrons. This results in a smaller proton gradient and consequently, less ATP production compared to aerobic respiration. This explains why aerobic respiration is significantly more energy-efficient than anaerobic respiration.
The Significance of Oxidative Phosphorylation and Oxygen's Role
The process of oxidative phosphorylation, driven by oxygen's role as the final electron acceptor, is vital for the survival of most organisms. It yields the vast majority of ATP generated during cellular respiration, providing the energy necessary for numerous cellular processes, including:
- Muscle contraction: The energy-demanding process of muscle movement relies heavily on ATP generated through oxidative phosphorylation.
- Active transport: Moving molecules against their concentration gradients across cell membranes requires ATP.
- Biosynthesis: The synthesis of macromolecules like proteins, nucleic acids, and lipids requires significant energy input.
- Cell signaling: Communication between cells often relies on energy-dependent processes.
- Maintenance of cellular integrity: Maintaining the structural integrity of cells requires energy.
The efficiency of oxidative phosphorylation is directly linked to the availability of oxygen. When oxygen is limited, organisms must rely on less efficient anaerobic pathways, resulting in reduced energy production and potentially leading to fatigue, cellular dysfunction, and even death in severe cases.
Evolutionary Implications and the Rise of Oxygen
The evolution of oxygenic photosynthesis, a process that releases oxygen as a byproduct, dramatically altered the course of life on Earth. The increasing availability of oxygen allowed for the evolution of more efficient aerobic respiration, leading to a significant increase in energy production. This higher energy yield likely fueled the evolution of larger, more complex organisms. Before the rise of oxygen, life relied primarily on anaerobic respiration, which is much less efficient.
Understanding the impact of oxygen deficiency
Oxygen deficiency, or hypoxia, can have severe consequences for various organisms. Humans, for example, can experience hypoxia-related issues including altitude sickness, reduced cognitive function, impaired athletic performance, and even death in extreme cases. In cells, hypoxia can lead to a shift towards anaerobic metabolism (fermentation), producing lactic acid as a byproduct which can lead to muscle fatigue and acidosis. The reduced ATP production due to the lack of oxygen can impair cellular function across various organ systems.
Conclusion
In summary, the final electron acceptor of aerobic cellular respiration is oxygen. This seemingly simple fact underpins a remarkably complex and crucial process – oxidative phosphorylation – that provides the vast majority of energy powering life as we know it. The unique properties of oxygen, including its high electronegativity and ability to readily accept electrons, allow for the efficient generation of ATP through the electron transport chain and chemiosmosis. Understanding the role of oxygen in cellular respiration is fundamental to appreciating the intricate interplay of biochemical pathways that maintain life and the profound evolutionary consequences of its emergence on our planet. Further research into the intricacies of oxidative phosphorylation, including the regulation of the ETC and its susceptibility to dysfunction, continues to be vital for advancing our understanding of health and disease.
Latest Posts
Latest Posts
-
Lowest Common Multiple Of 7 And 8
Mar 10, 2025
-
What Is The 15 Of 200
Mar 10, 2025
-
What Is 120 Minutes In Hours
Mar 10, 2025
-
How Many Quarts In 2 Cubic Feet
Mar 10, 2025
-
40 Inches Is How Many Feet
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about The Final Electron Acceptor Of Aerobic Cellular Respiration Is _____. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
