The Chemistry Of Living Organisms Is Called Chemistry
Juapaving
Mar 22, 2025 · 7 min read
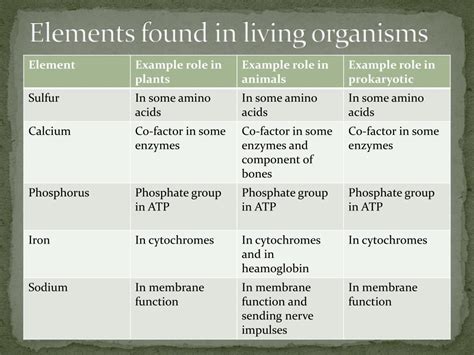
Table of Contents
The Chemistry of Living Organisms: A Deep Dive into Biochemistry
The chemistry of living organisms is called biochemistry. It's a vast and intricate field exploring the chemical processes within and relating to living organisms. From the smallest bacteria to the largest whales, life itself is fundamentally a complex interplay of chemical reactions. Understanding these reactions is crucial to comprehending how life functions, evolves, and responds to its environment. This article will delve into the core principles of biochemistry, exploring its major components and their significance.
The Building Blocks of Life: Biomolecules
Biochemistry centers around four major classes of biomolecules: carbohydrates, lipids, proteins, and nucleic acids. Each plays a unique and vital role in maintaining life.
1. Carbohydrates: The Energy Source
Carbohydrates are the primary source of energy for living organisms. They are composed of carbon, hydrogen, and oxygen atoms, usually in a ratio of 1:2:1. These molecules exist in various forms, ranging from simple sugars (monosaccharides like glucose and fructose) to complex polysaccharides (like starch and cellulose).
- Monosaccharides: These are the simplest carbohydrates, serving as the basic building blocks for larger molecules. Glucose, a crucial fuel for cellular respiration, is a prime example. Fructose, found in fruits, is another common monosaccharide.
- Disaccharides: Formed by the joining of two monosaccharides, these include sucrose (table sugar), lactose (milk sugar), and maltose (malt sugar). These are broken down into their constituent monosaccharides for energy utilization.
- Polysaccharides: These are complex carbohydrates composed of long chains of monosaccharides. Starch, a storage polysaccharide in plants, and glycogen, the animal equivalent, are essential energy reserves. Cellulose, a structural polysaccharide found in plant cell walls, provides rigidity and support. The diversity of polysaccharide structure significantly impacts their function.
2. Lipids: Structure, Storage, and Signaling
Lipids are a diverse group of hydrophobic (water-insoluble) biomolecules, including fats, oils, waxes, and steroids. They are primarily composed of carbon, hydrogen, and oxygen, but with a much lower proportion of oxygen than carbohydrates.
- Triglycerides: These are the most common type of lipid, serving as the primary energy storage molecules in animals. They consist of a glycerol molecule linked to three fatty acid chains. Saturated fatty acids have single bonds between carbon atoms, while unsaturated fatty acids contain one or more double bonds.
- Phospholipids: These are crucial components of cell membranes. They have a hydrophilic (water-loving) head and two hydrophobic tails, forming a bilayer structure that regulates the passage of substances into and out of the cell.
- Steroids: These are lipids with a characteristic four-ring structure. Cholesterol, a crucial component of animal cell membranes and a precursor for many hormones, is a prime example. Steroid hormones, like testosterone and estrogen, play vital roles in regulating various physiological processes.
3. Proteins: The Workhorses of the Cell
Proteins are the most diverse and versatile biomolecules, playing a myriad of roles in living organisms. They are composed of long chains of amino acids linked together by peptide bonds. The sequence of amino acids determines the protein's three-dimensional structure, which in turn dictates its function.
- Amino Acids: These are the building blocks of proteins, each with a unique side chain that influences the protein's properties. There are 20 standard amino acids used in protein synthesis.
- Protein Structure: Proteins exhibit four levels of structural organization: primary (amino acid sequence), secondary (local folding patterns like alpha-helices and beta-sheets), tertiary (overall three-dimensional structure), and quaternary (arrangement of multiple polypeptide chains). The specific structure dictates the protein's function.
- Protein Functions: Proteins perform a vast array of functions, including catalysis (enzymes), transport (hemoglobin), structural support (collagen), movement (actin and myosin), defense (antibodies), and signaling (hormones).
4. Nucleic Acids: The Information Carriers
Nucleic acids, DNA (deoxyribonucleic acid) and RNA (ribonucleic acid), are the carriers of genetic information. They are composed of nucleotides, each consisting of a sugar, a phosphate group, and a nitrogenous base.
- DNA: This double-stranded helix stores the genetic code, providing the instructions for building and maintaining an organism. The sequence of nucleotides in DNA dictates the sequence of amino acids in proteins.
- RNA: This molecule plays multiple roles in gene expression, including carrying the genetic information from DNA to ribosomes (mRNA), bringing amino acids to the ribosome during protein synthesis (tRNA), and forming part of the ribosome itself (rRNA).
Metabolism: The Chemical Processes of Life
Metabolism encompasses all the chemical reactions occurring within a living organism. These reactions are highly organized and regulated, ensuring the organism's survival and proper functioning.
1. Catabolism: Breaking Down Molecules
Catabolism involves the breakdown of complex molecules into simpler ones, releasing energy in the process. Cellular respiration, the breakdown of glucose to produce ATP (adenosine triphosphate), is a prime example. ATP is the main energy currency of the cell.
2. Anabolism: Building Up Molecules
Anabolism involves the synthesis of complex molecules from simpler ones, requiring energy input. Protein synthesis, the creation of proteins from amino acids, and the synthesis of polysaccharides from monosaccharides are examples of anabolic processes.
Enzymes: The Catalysts of Life
Enzymes are biological catalysts, mostly proteins, that accelerate the rate of biochemical reactions without being consumed themselves. They achieve this by lowering the activation energy required for a reaction to occur. The specificity of enzyme action is crucial for the precise regulation of metabolic pathways.
- Enzyme-Substrate Interaction: Enzymes bind to specific substrates (reactant molecules) at their active site, forming an enzyme-substrate complex. This interaction facilitates the reaction, leading to the formation of products.
- Enzyme Regulation: Enzyme activity is tightly regulated to ensure that metabolic pathways operate efficiently and in response to the organism's needs. Regulation can occur through various mechanisms, including allosteric regulation, covalent modification, and feedback inhibition.
The Importance of Biochemistry
Understanding biochemistry is crucial for numerous reasons:
- Medicine: Biochemistry underpins our understanding of diseases and the development of new treatments. Many diseases are caused by defects in biochemical pathways, and drug development often targets specific enzymes or metabolic processes.
- Agriculture: Biochemistry plays a crucial role in improving crop yields and developing pest-resistant plants. Understanding plant metabolism allows for the development of more efficient agricultural practices.
- Environmental Science: Biochemistry helps us understand the impact of pollutants on living organisms and develop strategies for environmental remediation. Bioremediation, the use of microorganisms to clean up pollutants, relies on understanding biochemical processes.
- Biotechnology: Biochemistry is fundamental to biotechnology, enabling the development of new technologies for producing pharmaceuticals, biofuels, and other valuable products. Genetic engineering and metabolic engineering rely heavily on biochemical principles.
Future Directions in Biochemistry
Biochemistry continues to evolve rapidly, with several exciting areas of research:
- Systems Biology: This field integrates various "omics" approaches (genomics, proteomics, metabolomics) to understand the complex interactions between different components of a biological system.
- Synthetic Biology: This field aims to design and build new biological systems or redesign existing ones to perform novel functions. This involves engineering metabolic pathways and genetic circuits.
- Structural Biology: Determining the three-dimensional structures of biomolecules using techniques like X-ray crystallography and NMR spectroscopy helps to elucidate their functions and interactions.
- Computational Biochemistry: Using computational methods to model and simulate biochemical processes is becoming increasingly important for understanding complex systems and predicting the effects of mutations or drug interactions.
In conclusion, biochemistry is a cornerstone of modern biology and medicine. Its intricate and diverse nature reflects the complexity of life itself. From understanding the basic building blocks of life to deciphering the complex metabolic pathways that sustain it, biochemistry continues to provide profound insights into the workings of living organisms and holds immense promise for solving some of humanity's most pressing challenges. Continued exploration in this field will undoubtedly lead to further advancements in our understanding of life and pave the way for novel applications across various scientific and technological domains. The study of biochemistry is not just a scientific pursuit; it is an exploration into the very essence of what makes life possible.
Latest Posts
Latest Posts
-
Why Is The Cell Membrane Called Selectively Permeable
Mar 23, 2025
-
What Is The Prime Factorization For 125
Mar 23, 2025
-
Which Of The Following Is The Best Conductor Of Heat
Mar 23, 2025
-
How Do Ferns And Mosses Reproduce
Mar 23, 2025
-
Is Energy Conserved In Elastic Collisions
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about The Chemistry Of Living Organisms Is Called Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
