Select Three Components That Make Up A Nucleotide
Juapaving
Mar 23, 2025 · 7 min read
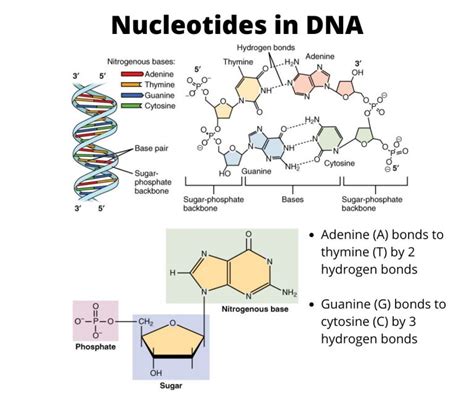
Table of Contents
Decoding the Nucleotide: The Three Essential Components
Nucleotides, the fundamental building blocks of DNA and RNA, are far more than just simple molecules. Understanding their structure is key to grasping the complexities of genetics and molecular biology. This in-depth exploration will delve into the three crucial components that constitute a nucleotide: the nitrogenous base, the pentose sugar, and the phosphate group. We'll examine each component individually, highlighting their unique properties and their collective contribution to the overall structure and function of nucleotides.
1. The Nitrogenous Base: The Information Carrier
The nitrogenous base is the heart of the nucleotide, responsible for carrying the genetic information. These are cyclic organic molecules containing nitrogen atoms, and they come in two main categories: purines and pyrimidines. This categorization is crucial because it directly impacts the structure and base-pairing capabilities of DNA and RNA.
Purines: Adenine (A) and Guanine (G)
Purines are characterized by a double-ring structure, comprising a six-membered ring fused to a five-membered ring. Two purines, adenine (A) and guanine (G), are found in both DNA and RNA. Their larger size plays a crucial role in their interaction with pyrimidines during base pairing. The specific arrangement of atoms within their ring structures allows for the formation of hydrogen bonds with their complementary pyrimidine bases.
-
Adenine (A): Adenine features an amino group (-NH2) at the 6-position of its ring system. This amino group's ability to participate in hydrogen bonding is fundamental to its base-pairing function with thymine (in DNA) or uracil (in RNA).
-
Guanine (G): Guanine possesses a keto group (=O) at the 6-position and an amino group (-NH2) at the 2-position. This specific arrangement allows for the formation of three hydrogen bonds with its complementary base, cytosine.
Pyrimidines: Cytosine (C), Thymine (T), and Uracil (U)
Pyrimidines are characterized by a single six-membered ring structure. Three pyrimidines—cytosine (C), thymine (T), and uracil (U)—participate in the formation of nucleotides.
-
Cytosine (C): Cytosine, found in both DNA and RNA, features an amino group (-NH2) at the 4-position and a keto group (=O) at the 2-position. This configuration allows for three hydrogen bonds to form with guanine.
-
Thymine (T): Thymine is found exclusively in DNA. It features two keto groups (=O) at the 2- and 4-positions, along with a methyl group (-CH3) at the 5-position. This methyl group distinguishes thymine from uracil and contributes to its unique base-pairing properties with adenine.
-
Uracil (U): Uracil is the pyrimidine found only in RNA. It is essentially a demethylated form of thymine, lacking the methyl group at the 5-position. This difference results in a slightly weaker interaction with adenine compared to the thymine-adenine interaction in DNA.
The precise sequence of these nitrogenous bases within a DNA or RNA molecule dictates the genetic code, determining the synthesis of proteins and the overall functionality of an organism. The specific properties of each base are essential for the accurate replication and transcription of genetic information. The hydrogen bonding between complementary bases is the foundation of the double helix structure of DNA and the crucial step in many genetic processes.
2. The Pentose Sugar: The Structural Backbone
The second key component of a nucleotide is the pentose sugar, a five-carbon sugar molecule that provides the structural framework to which the nitrogenous base and phosphate group are attached. The sugar's identity—either ribose or deoxyribose—differentiates DNA from RNA.
Ribose in RNA
In RNA (ribonucleic acid), the sugar is ribose, a pentose sugar with a hydroxyl group (-OH) attached to the 2' carbon atom. This hydroxyl group's presence contributes to RNA's higher reactivity and susceptibility to hydrolysis compared to DNA. RNA’s single-stranded structure, in part due to the presence of this hydroxyl group, allows for greater flexibility and a wider range of functional roles beyond simply storing genetic information. These roles include acting as messenger RNA (mRNA), transfer RNA (tRNA), and ribosomal RNA (rRNA), all crucial in protein synthesis.
Deoxyribose in DNA
In DNA (deoxyribonucleic acid), the sugar is deoxyribose, a modified form of ribose lacking a hydroxyl group (-OH) at the 2' carbon atom. This absence of the hydroxyl group makes DNA more stable and less prone to hydrolysis than RNA. This greater stability is crucial for DNA's role as the long-term storage molecule for genetic information. The stability of the double helix structure, maintained through base pairing and the deoxyribose backbone, is essential for protecting the integrity of the genome.
The pentose sugar, through its 1' carbon, provides the crucial attachment point for the nitrogenous base, forming a nucleoside. The linkage between the sugar and base is a glycosidic bond, a crucial structural feature that contributes to the overall stability and three-dimensional shape of the nucleotide. The configuration of this bond, whether α or β, also influences the molecule's properties.
3. The Phosphate Group: The Energy Source and Linker
The third essential component of a nucleotide is the phosphate group (PO43-), a negatively charged group consisting of a phosphorus atom bonded to four oxygen atoms. The phosphate group's role is multifaceted. Firstly, it plays a vital role in energy transfer within the cell. Secondly, it links nucleotides together to form the polynucleotide chains of DNA and RNA.
High-Energy Phosphate Bonds
The phosphate bonds in nucleotides are high-energy bonds, meaning that their hydrolysis (breaking down with water) releases a significant amount of energy. This energy is harnessed by the cell to drive various metabolic processes. Adenosine triphosphate (ATP), a nucleotide composed of adenine, ribose, and three phosphate groups, is the primary energy currency of cells. The hydrolysis of ATP to ADP (adenosine diphosphate) releases energy that fuels numerous cellular activities, including muscle contraction, active transport, and biosynthesis.
Linking Nucleotides to Form Polynucleotides
The phosphate group also plays a crucial role in connecting individual nucleotides to create long chains, known as polynucleotides. This linkage occurs through a phosphodiester bond, where the phosphate group forms a bridge between the 3' carbon of one sugar and the 5' carbon of the next sugar. This creates the sugar-phosphate backbone of DNA and RNA, which runs antiparallel—one strand running 5' to 3' while its complement runs 3' to 5'. This antiparallel arrangement is critical for the double helix structure of DNA and contributes significantly to its stability and function. The phosphodiester bonds' stability ensures the integrity of the genetic information encoded within the nucleotide sequence.
Nucleotide Variations and Functions
The combination of these three components—the nitrogenous base, the pentose sugar, and the phosphate group—results in an incredible diversity of nucleotides. The specific nitrogenous base determines the nucleotide's identity (A, T, C, G, or U), and whether it is found in DNA or RNA. The pentose sugar (ribose or deoxyribose) further differentiates DNA and RNA, influencing their stability and functions. The number of phosphate groups attached can also vary, as seen in molecules like ATP, ADP, and AMP. These variations contribute to the wide range of functions of nucleotides, from energy storage and transfer to information storage and protein synthesis.
Conclusion: The Interplay of Components
The three components of a nucleotide—the nitrogenous base, the pentose sugar, and the phosphate group—are intricately interconnected and essential for the nucleotide's function. The nitrogenous base carries the genetic information, the pentose sugar provides the structural backbone, and the phosphate group provides energy and links nucleotides together to form the polynucleotide chains of DNA and RNA. The precise arrangement and interactions of these components determine the overall structure, stability, and functionality of DNA and RNA, highlighting the remarkable elegance and sophistication of these fundamental biological molecules. Understanding the individual roles of each component is paramount to grasping the complexities of life at a molecular level. The study of nucleotides and their function continues to be a crucial area of research with far-reaching implications for understanding genetics, disease, and the development of new therapies.
Latest Posts
Latest Posts
-
Which Components Are Necessary For Photosynthesis To Occur
Mar 24, 2025
-
When Solutions Of Nacl And Agno3 Are Mixed
Mar 24, 2025
-
As The Wavelength Of Light Increases The Frequency
Mar 24, 2025
-
What Will Not Dissolve In Water
Mar 24, 2025
-
Compare And Contrast Convection And Conduction
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Select Three Components That Make Up A Nucleotide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
