Salt Is A Compound Or Element
Juapaving
Mar 14, 2025 · 5 min read
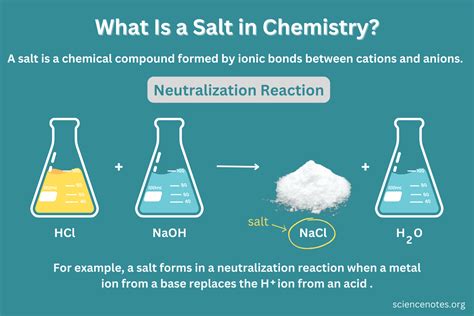
Table of Contents
Salt: Compound, Not Element – A Deep Dive into Chemical Composition
Salt, a ubiquitous substance found in our kitchens and playing a vital role in numerous industrial processes, is often mistakenly considered an element. However, salt, specifically table salt or sodium chloride (NaCl), is a compound, not an element. This article will delve deep into the chemical composition of salt, explaining why it's classified as a compound, its properties, and its significance in various contexts. We’ll also explore related concepts to provide a comprehensive understanding of this fundamental chemical substance.
Understanding Elements and Compounds
Before we delve into the specifics of salt, it's crucial to define the fundamental terms: element and compound.
What is an Element?
An element is a pure substance consisting only of atoms that all have the same number of protons in their atomic nuclei. These are the fundamental building blocks of all matter. Elements are listed in the periodic table, and each is represented by a unique chemical symbol (e.g., H for hydrogen, O for oxygen, Na for sodium, Cl for chlorine). Elements cannot be broken down into simpler substances by chemical means.
What is a Compound?
A compound, on the other hand, is a substance formed when two or more chemical elements are chemically bonded together. These bonds can be ionic (like in salt) or covalent (like in water). Compounds have different properties than the elements that constitute them. They can be broken down into their constituent elements through chemical reactions.
Salt: A Chemical Compound
Now, let's focus on salt, specifically sodium chloride (NaCl). Table salt is an ionic compound formed from the electrostatic attraction between positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-). This means that sodium atoms lose an electron to become positively charged ions, and chlorine atoms gain an electron to become negatively charged ions. This transfer of electrons forms an ionic bond, holding the ions together in a crystal lattice structure.
The Ionic Bond in NaCl
The ionic bond in NaCl is a strong electrostatic attraction. The opposite charges of the sodium and chloride ions attract each other, forming a stable, crystalline structure. This is why salt forms cubic crystals – the structure that minimizes the electrostatic energy of the ions. This strong ionic bond is responsible for many of salt's physical properties, such as its high melting point and solubility in water.
Why Salt is Not an Element
Since salt is composed of two different elements, sodium (Na) and chlorine (Cl), chemically bonded together, it inherently cannot be an element. Elements are pure substances consisting of only one type of atom. Salt, by its very nature of being a combination of sodium and chlorine atoms, fulfills the definition of a compound.
Properties of Salt (NaCl)
Understanding the properties of salt helps solidify its classification as a compound.
Physical Properties:
- Crystalline Structure: Salt exists as a crystalline solid, forming cubic crystals due to the arrangement of ions in its lattice.
- High Melting Point: The strong ionic bonds require a significant amount of energy to break, resulting in a high melting point (around 801°C).
- Solubility in Water: Salt readily dissolves in water because the polar water molecules interact strongly with the charged sodium and chloride ions, breaking the ionic bonds and allowing the ions to become hydrated.
- Brittleness: Salt crystals are brittle because the layers of ions can easily slide past each other, causing the crystal to fracture.
- White Color: Pure NaCl is white, although impurities can impart different colors.
Chemical Properties:
- Ionic Nature: The most defining chemical property is its ionic nature, resulting from the transfer of electrons between sodium and chlorine atoms.
- Reactivity with Metals: Salt can react with certain metals to displace them from their compounds.
- Reaction with Acids and Bases: Salt can react with acids and bases to form new salts and water.
- Electrolysis: When dissolved in water and subjected to electrolysis, salt can be broken down into its constituent elements, sodium and chlorine. This demonstrates its compound nature, as elements cannot be broken down chemically into simpler substances.
The Importance of Salt
Salt's significance extends far beyond its culinary applications.
Culinary Uses:
Salt is essential in food preparation for flavor enhancement, preservation, and as a crucial component in many recipes. Its ability to enhance flavors and preserve food has been crucial throughout human history.
Industrial Applications:
- De-icing Roads: Salt's ability to lower the freezing point of water is widely used for de-icing roads and pavements during winter.
- Water Softening: Salt is used in water softening systems to remove calcium and magnesium ions from hard water.
- Chemical Industry: Salt is a raw material for the production of various chemicals, including sodium hydroxide (NaOH), chlorine gas (Cl2), and sodium carbonate (Na2CO3). These chemicals are fundamental in countless industrial processes.
- Medicine: Sodium chloride is an essential electrolyte and is used in intravenous solutions to maintain fluid balance in patients.
Biological Importance:
Sodium and chloride ions are crucial for various biological processes. They are vital for nerve impulse transmission, muscle contraction, and maintaining fluid balance in living organisms.
Distinguishing Salt from Other Compounds
While table salt (NaCl) is the most common type of salt, it’s important to note that the term "salt" in chemistry refers to a broader class of ionic compounds formed from the reaction of an acid and a base. These compounds are often called ionic salts or simply salts. Examples include potassium chloride (KCl), calcium chloride (CaCl2), and ammonium nitrate (NH4NO3). Each of these compounds has unique properties, differing from NaCl in many aspects, emphasizing the diversity within the category of "salts."
Conclusion
In conclusion, salt, specifically sodium chloride (NaCl), is unequivocally a compound, not an element. Its composition of two distinct elements, sodium and chlorine, chemically bonded through an ionic bond, distinctly classifies it as a compound. Understanding this fundamental difference is crucial for grasping basic chemistry principles. The properties of salt, both physical and chemical, are a direct result of its ionic nature and composition. Its widespread uses in culinary practices, various industries, and biological processes underscore its importance in our daily lives. The detailed analysis presented here reinforces the understanding of the distinction between elements and compounds, providing a strong foundation for further exploration of chemical concepts. Understanding the distinction between an element and a compound is a cornerstone of chemical knowledge.
Latest Posts
Latest Posts
-
Which Number Is A Multiple Of 6 And 8
Mar 14, 2025
-
Why Are Fossils Found Mostly In Sedimentary Rocks
Mar 14, 2025
-
What Is The Difference Between Open And Closed Circulatory Systems
Mar 14, 2025
-
10 Is A Multiple Of 5
Mar 14, 2025
-
How Many Edges Are There In A Rectangular Prism
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Salt Is A Compound Or Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
