Salicylic Acid And Acetic Anhydride Reaction
Juapaving
Mar 13, 2025 · 5 min read
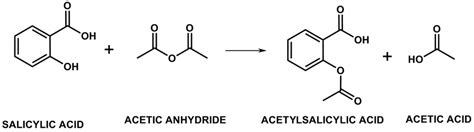
Table of Contents
Salicylic Acid and Acetic Anhydride: A Deep Dive into the Synthesis of Aspirin
The synthesis of aspirin, or acetylsalicylic acid, from salicylic acid and acetic anhydride is a classic organic chemistry experiment. This reaction, an esterification, is relatively simple to perform yet offers a fascinating glimpse into the world of chemical transformations and reaction mechanisms. This comprehensive guide will explore the reaction in detail, covering its mechanism, reaction conditions, purification techniques, and safety considerations. We'll also delve into the broader applications and significance of both salicylic acid and aspirin.
Understanding the Reaction: Esterification of Salicylic Acid
The core of aspirin synthesis lies in the esterification of salicylic acid with acetic anhydride. Salicylic acid, a naturally occurring phenolic compound, possesses both a carboxylic acid group (-COOH) and a hydroxyl group (-OH) on its aromatic ring. Acetic anhydride is a reactive derivative of acetic acid, providing the acetyl group (-COCH₃) necessary to form the ester linkage.
The Reaction Equation:
The reaction can be summarized by the following equation:
Salicylic Acid + Acetic Anhydride ⇌ Acetylsalicylic Acid (Aspirin) + Acetic Acid
This is an equilibrium reaction, meaning it proceeds in both the forward and reverse directions. To drive the equilibrium towards the formation of aspirin, we employ certain strategies, which we'll discuss later.
The Mechanism: A Step-by-Step Breakdown
The esterification reaction proceeds via an acid-catalyzed nucleophilic acyl substitution mechanism. A strong acid catalyst, typically sulfuric acid or phosphoric acid, is crucial for this reaction. Here's a breakdown of the steps:
-
Protonation of Acetic Anhydride: The acid catalyst protonates the carbonyl oxygen of acetic anhydride, increasing its electrophilicity. This makes the anhydride molecule more susceptible to nucleophilic attack.
-
Nucleophilic Attack by Salicylic Acid: The hydroxyl group (-OH) of salicylic acid, acting as a nucleophile, attacks the electrophilic carbonyl carbon of the protonated acetic anhydride. This forms a tetrahedral intermediate.
-
Acetate Ion Departure: The tetrahedral intermediate is unstable. One of the acetate groups leaves as an acetate ion, regenerating a carbonyl group.
-
Deprotonation: The protonated hydroxyl group on the newly formed acetyl group loses a proton, yielding the aspirin molecule and acetic acid.
-
Regeneration of the Catalyst: The acetate ion accepts a proton from the solution, regenerating the acid catalyst.
Optimizing the Reaction: Factors Influencing Yield and Purity
Several factors significantly influence the yield and purity of the synthesized aspirin:
1. Catalyst Choice and Concentration: The choice and concentration of the acid catalyst are critical. Sulfuric acid is a common choice due to its strong acidity; however, phosphoric acid is a safer alternative. An appropriate concentration must be used; excessive acid can lead to side reactions, while insufficient acid will result in a low yield.
2. Temperature: The reaction is typically carried out at a slightly elevated temperature (around 50-60°C). Higher temperatures can accelerate the reaction but might also lead to decomposition or unwanted side reactions.
3. Reaction Time: Sufficient reaction time is essential to allow the reaction to reach equilibrium. Typically, a reaction time of 15-20 minutes is sufficient.
4. Stoichiometry: Using an excess of acetic anhydride helps drive the equilibrium towards the formation of aspirin and increases the yield.
5. Purification Techniques: Purification is critical to obtain high-purity aspirin. Recrystallization is commonly employed, involving dissolving the crude product in a hot solvent (e.g., ethanol or a mixture of ethanol and water) and allowing it to slowly cool and crystallize. The purified aspirin crystals are then filtered and dried.
Safety Considerations: Handling Chemicals with Care
Working with chemicals requires meticulous attention to safety protocols. Here are essential safety precautions for aspirin synthesis:
- Eye Protection: Always wear safety goggles to protect your eyes from splashes.
- Gloves: Wear chemical-resistant gloves to prevent skin contact with the chemicals.
- Fume Hood: Conduct the reaction in a fume hood to avoid inhaling any harmful vapors.
- Proper Disposal: Dispose of all waste materials according to the appropriate safety guidelines. Acetic acid and acetic anhydride are corrosive, and sulfuric acid is highly corrosive and hazardous.
- Avoid Ingestion: Never ingest any chemicals during the experiment.
Applications of Salicylic Acid and Aspirin: Beyond the Lab
Both salicylic acid and aspirin have far-reaching applications, spanning various fields:
Salicylic Acid:
- Medicine: Salicylic acid is a key ingredient in many topical medications for acne, warts, and psoriasis. Its keratolytic properties help shed dead skin cells.
- Cosmetics: It's found in many skincare products due to its exfoliating and anti-inflammatory properties.
- Food Preservative: In low concentrations, salicylic acid can act as a food preservative.
Aspirin (Acetylsalicylic Acid):
- Pain Relief: Aspirin is a widely used analgesic (pain reliever) and antipyretic (fever reducer).
- Anti-inflammatory: It possesses anti-inflammatory properties, making it effective for treating conditions like arthritis.
- Antiplatelet Agent: Aspirin inhibits platelet aggregation, reducing the risk of blood clots and stroke. This is particularly relevant in preventing cardiovascular events.
- Cancer Research: Research indicates potential anticancer properties of aspirin, although more research is needed.
Conclusion: A Classic Reaction with Enduring Significance
The synthesis of aspirin from salicylic acid and acetic anhydride remains a crucial experiment in organic chemistry education. It demonstrates fundamental concepts like esterification, reaction mechanisms, and purification techniques. Furthermore, understanding this reaction provides valuable insight into the properties and applications of both salicylic acid and aspirin, two compounds with significant impacts on medicine, cosmetics, and other industries. By understanding the intricacies of this synthesis, we appreciate the power of chemical transformations and the significant contributions of chemistry to improving human health and well-being. The careful planning, precise execution, and rigorous safety measures involved in this synthesis highlight the importance of responsible scientific practice. While seemingly simple, this reaction embodies the complexity and elegance of chemical processes, reinforcing the vital role of chemistry in our world. Further research into optimizing the reaction yield and exploring potential modifications continue to expand our understanding of this fundamental chemical process. The continued exploration of salicylic acid and aspirin’s properties and applications promise further advancements in medicine and other fields.
Latest Posts
Latest Posts
-
What Is A Male Honeybee Called
May 09, 2025
-
What Is The Difference Between Antiseptic And Antibiotic
May 09, 2025
-
How Fast Is 90 Km An Hour
May 09, 2025
-
The Time Of Maximum Daily Temperature Occurs
May 09, 2025
-
Matter Includes All Of The Following Except
May 09, 2025
Related Post
Thank you for visiting our website which covers about Salicylic Acid And Acetic Anhydride Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.