Relation Between Mass Volume And Density
Juapaving
Mar 17, 2025 · 7 min read
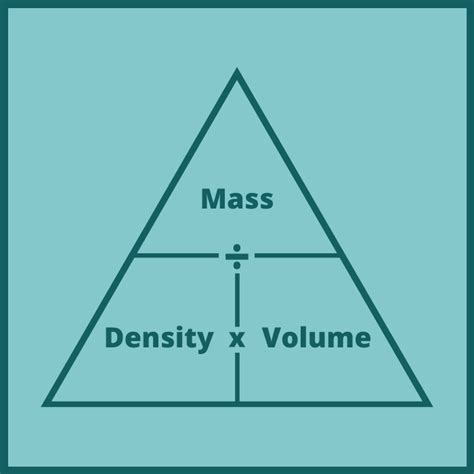
Table of Contents
The Intimate Relationship Between Mass, Volume, and Density
Understanding the relationship between mass, volume, and density is fundamental to numerous scientific disciplines, from physics and chemistry to engineering and geology. This seemingly simple concept underpins a wide range of applications, from designing efficient aircraft to understanding the formation of stars. This article will delve deep into this relationship, exploring the definitions of each term, their interdependencies, and real-world applications. We'll also discuss how to calculate density and the various methods used to measure mass and volume.
Defining the Key Terms: Mass, Volume, and Density
Before we explore their interconnectedness, let's clearly define each term:
Mass
Mass is a measure of the amount of matter in an object. It's a scalar quantity, meaning it only has magnitude and no direction. A common misconception is to confuse mass with weight. While weight is the force exerted on an object due to gravity, mass remains constant regardless of gravitational pull. For instance, an astronaut has the same mass on the moon as they do on Earth, even though their weight is significantly less due to the moon's weaker gravity. The standard unit for mass in the International System of Units (SI) is the kilogram (kg).
Volume
Volume is the amount of three-dimensional space occupied by an object or substance. It's also a scalar quantity, measured in cubic meters (m³) in the SI system. Other common units include liters (L), milliliters (mL), cubic centimeters (cm³), and gallons (gal). Determining volume depends on the object's shape. Regularly shaped objects, like cubes or spheres, have easily calculated volumes using geometric formulas. Irregularly shaped objects require displacement methods, often involving submerging the object in water and measuring the increase in water level.
Density
Density is the measure of how much mass is contained within a given volume. It's a crucial property that distinguishes different substances. A higher density signifies that more mass is packed into a smaller volume, while a lower density means the mass is more spread out. The formula for density is:
Density (ρ) = Mass (m) / Volume (V)
The SI unit for density is kilograms per cubic meter (kg/m³). Other common units include grams per cubic centimeter (g/cm³) and grams per milliliter (g/mL). It's important to note that density is temperature-dependent; most substances expand when heated, leading to a decrease in density.
The Interplay of Mass, Volume, and Density
The formula above beautifully encapsulates the relationship between mass, volume, and density. This simple equation allows us to calculate any one of these quantities if the other two are known. This interplay has profound implications across various scientific fields:
-
Material Identification: Density is a characteristic property of a substance. Knowing the density of an unknown material can often help identify it. For example, gold has a much higher density than iron, allowing for easy distinction.
-
Fluid Dynamics: Density plays a pivotal role in understanding fluid behavior. Buoyancy, for example, is directly related to the density difference between an object and the fluid it's submerged in. Archimedes' principle illustrates this, stating that an object submerged in a fluid experiences an upward buoyant force equal to the weight of the fluid displaced.
-
Meteorology: Atmospheric density influences weather patterns. Changes in air density due to temperature and pressure variations create pressure gradients, driving wind and affecting atmospheric circulation.
-
Astronomy: Density is crucial in understanding the formation and evolution of stars and planets. The density of a star determines its gravitational pull and its eventual fate.
-
Engineering: Density considerations are vital in engineering design. For example, choosing materials with appropriate densities is essential in designing lightweight yet strong structures for aircraft or spacecraft.
Calculating Density: Practical Examples
Let's illustrate the calculation of density with a few examples:
Example 1: A cube of iron
Imagine a cube of iron with a mass of 7.87 kg and side length of 10 cm. To calculate its density:
-
Calculate the volume: The volume of a cube is side length cubed (V = s³). In this case, V = (10 cm)³ = 1000 cm³.
-
Convert units (if necessary): It's crucial to use consistent units. Let's convert the volume to cubic meters: 1000 cm³ * (1 m/100 cm)³ = 0.001 m³.
-
Apply the density formula: ρ = m/V = 7.87 kg / 0.001 m³ = 7870 kg/m³. This is consistent with the known density of iron.
Example 2: An irregularly shaped object
Let's say we have an irregularly shaped rock. To determine its density, we'll use the water displacement method:
-
Measure the mass: Weigh the rock using a balance scale. Let's say the mass is 250 g.
-
Measure the volume: Fill a graduated cylinder with a known volume of water (e.g., 50 mL). Submerge the rock completely. The increase in water level represents the rock's volume. Let's say the water level rises to 75 mL. Therefore, the rock's volume is 75 mL - 50 mL = 25 mL. Remember to convert mL to cm³ (1 mL = 1 cm³).
-
Apply the density formula: ρ = m/V = 250 g / 25 cm³ = 10 g/cm³.
Methods for Measuring Mass and Volume
Accurate measurement of mass and volume is crucial for precise density calculations. Various methods are employed depending on the object's characteristics and the required accuracy:
Mass Measurement
-
Balance Scales: These provide accurate mass measurements by comparing the object's weight to known standards. Different types of balances exist, from simple beam balances to sophisticated analytical balances capable of measuring to microgram precision.
-
Electronic Balances: These utilize strain gauges or electromagnetic force to measure mass quickly and accurately. They are widely used in various settings due to their convenience and precision.
Volume Measurement
-
Graduated Cylinders: These are cylindrical containers with markings indicating volume. They are commonly used for measuring the volume of liquids and for the water displacement method for irregularly shaped objects.
-
Burets: These are precisely calibrated glass tubes used for delivering accurate volumes of liquids, particularly in titrations.
-
Pipets: These are used for transferring precise volumes of liquids. Various types exist, including volumetric pipets and graduated pipets.
-
Displacement Method: As previously mentioned, this method involves submerging an object in a liquid and measuring the change in volume. This is especially useful for irregularly shaped solids.
-
Geometric Formulas: For regularly shaped objects (cubes, spheres, cylinders, etc.), simple geometric formulas can be used to calculate the volume precisely.
Density and its Applications in Different Fields
The concept of density extends far beyond simple calculations. Its applications are diverse and crucial in various scientific and engineering disciplines:
Geology and Geophysics:
Density plays a vital role in understanding the Earth's structure. The varying densities of the Earth's layers (crust, mantle, core) are responsible for its gravitational field and plate tectonics. Geophysicists use density measurements to map subsurface structures and explore for mineral resources.
Materials Science:
Understanding the density of materials is essential in material selection for engineering applications. Choosing materials with specific density requirements is crucial for optimizing structural performance and weight considerations in designing airplanes, cars, and buildings. The relationship between density and other material properties, such as strength and stiffness, is crucial for material selection.
Oceanography:
Oceanographic studies rely heavily on density measurements to understand ocean currents, water stratification, and the distribution of marine organisms. Density variations drive thermohaline circulation, a crucial global ocean current system impacting climate.
Medicine:
Density measurements are used in medical imaging techniques, such as bone densitometry, which helps diagnose osteoporosis. The density of tissues and organs can provide valuable information for medical diagnoses.
Conclusion: A Fundamental Concept with Wide-Reaching Implications
The relationship between mass, volume, and density is a fundamental concept in science and engineering. Understanding this relationship is key to comprehending a vast range of phenomena, from the behavior of fluids to the structure of the Earth. The ability to accurately measure mass and volume, and to calculate density using the simple formula, ρ = m/V, provides a powerful tool for investigating the physical world around us. This article has explored the definitions, the interconnectedness, the methods of measurement, and the broad applications of these fundamental concepts. The understanding and application of these principles are critical for advancements in various fields and will continue to play an essential role in scientific discovery and technological innovation for years to come.
Latest Posts
Latest Posts
-
Lowest Common Multiple Of 28 And 32
Mar 17, 2025
-
What Are The Factor Pairs Of 30
Mar 17, 2025
-
The Idea Of Spontaneous Generation Postulated That
Mar 17, 2025
-
How Many Sides Are There In A Pentagon
Mar 17, 2025
-
How Are Mixtures Different From Solutions
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Relation Between Mass Volume And Density . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
