Reaction Of Salicylic Acid With Acetic Anhydride
Juapaving
Mar 11, 2025 · 6 min read
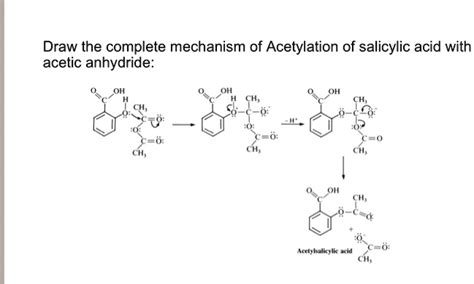
Table of Contents
The Reaction of Salicylic Acid with Acetic Anhydride: A Comprehensive Guide
The synthesis of aspirin (acetylsalicylic acid) from salicylic acid and acetic anhydride is a classic organic chemistry experiment, illustrating the principles of esterification. This reaction is not only crucial for understanding fundamental chemical concepts but also holds immense practical significance due to aspirin's widespread use as an analgesic and anti-inflammatory drug. This detailed guide will explore every facet of this reaction, from the mechanism to practical considerations for achieving a high yield and purity.
Understanding the Reactants: Salicylic Acid and Acetic Anhydride
Before delving into the reaction itself, let's examine the properties of the reactants:
Salicylic Acid: The Starting Material
Salicylic acid (2-hydroxybenzoic acid) is a naturally occurring phenolic compound found in willow bark. Its structure features both a carboxylic acid (-COOH) group and a hydroxyl (-OH) group on the benzene ring. The hydroxyl group is crucial for the reaction with acetic anhydride, as it acts as the nucleophile. Salicylic acid is a white crystalline solid, slightly soluble in water but more soluble in organic solvents like ethanol.
Acetic Anhydride: The Acylating Agent
Acetic anhydride is an organic compound with the formula (CH₃CO)₂O. It acts as the acylating agent in this reaction, providing the acetyl group (-COCH₃) which will replace the hydrogen atom of the hydroxyl group in salicylic acid. It is a colorless liquid with a pungent vinegar-like odor, slightly soluble in water, and readily soluble in organic solvents. Acetic anhydride is a more reactive acylating agent compared to acetic acid, making it suitable for this esterification reaction.
The Esterification Reaction: Mechanism and Kinetics
The reaction between salicylic acid and acetic anhydride is an example of an esterification reaction, specifically an acetylation. This process involves the formation of an ester bond (-COO-) between the salicylic acid and acetic anhydride. The reaction mechanism can be described as follows:
Step-by-Step Mechanism:
-
Nucleophilic Attack: The hydroxyl group (-OH) of salicylic acid, acting as a nucleophile, attacks the electrophilic carbonyl carbon of acetic anhydride. This forms a tetrahedral intermediate.
-
Proton Transfer: A proton is transferred from the hydroxyl group of the tetrahedral intermediate to one of the acetate groups.
-
Elimination: The acetate group leaves as acetate ion, forming the acetylsalicylic acid (aspirin) molecule and acetic acid as a byproduct.
Reaction Kinetics:
The reaction is typically carried out in the presence of a catalyst, often sulfuric acid or phosphoric acid. The catalyst accelerates the reaction by protonating the carbonyl oxygen of acetic anhydride, making it a more potent electrophile, thereby enhancing the rate of nucleophilic attack by the hydroxyl group of salicylic acid. The reaction rate is influenced by several factors:
-
Temperature: Higher temperatures generally increase the reaction rate, but excessively high temperatures can lead to decomposition of reactants or products.
-
Catalyst Concentration: A suitable catalyst concentration is crucial. Too little catalyst may slow down the reaction, while too much can lead to side reactions or decomposition.
-
Reactant Concentrations: The reaction rate is directly proportional to the concentrations of both salicylic acid and acetic anhydride.
-
Solvent: The choice of solvent can significantly impact the reaction rate and yield.
Practical Considerations for Synthesis:
Optimizing the aspirin synthesis involves careful consideration of several factors:
Choice of Catalyst:
Sulfuric acid and phosphoric acid are common catalysts. Phosphoric acid is preferred in many cases due to its lower corrosiveness and ease of handling compared to sulfuric acid. The amount of catalyst used should be carefully controlled to achieve the desired reaction rate without causing unwanted side reactions.
Reaction Conditions:
The reaction is typically carried out at a moderately elevated temperature (around 50-60°C). This temperature range provides a balance between reaction rate and minimizing side reactions or product degradation. The reaction is usually conducted in a water bath to maintain a consistent temperature.
Purification of Aspirin:
The crude aspirin product obtained after the reaction needs purification to remove impurities such as unreacted salicylic acid, acetic acid, and the catalyst. Recrystallization is a commonly used technique. This involves dissolving the crude aspirin in a hot solvent (usually ethanol or a mixture of ethanol and water), allowing the solution to cool slowly, and then filtering the resulting crystals. The purified aspirin is usually white crystalline solid.
Determining the Yield and Purity:
The yield of aspirin is calculated by comparing the actual amount of aspirin obtained to the theoretical yield based on the starting amount of salicylic acid. The purity of the synthesized aspirin can be determined using various techniques, including melting point determination, and thin-layer chromatography (TLC).
Advanced Considerations and Variations:
Green Chemistry Approaches:
Modern approaches emphasize greener and more sustainable methods. For example, using alternative catalysts, such as solid acid catalysts, which are easier to separate and potentially more environmentally benign, are actively researched. Solvent-free reactions, eliminating the use of potentially harmful organic solvents, are also being explored.
Scale-up of the Reaction:
The reaction can be scaled up for larger-scale production of aspirin. However, this requires careful consideration of safety, handling, and efficient mixing and heat transfer to maintain uniform reaction conditions throughout the larger reaction vessel.
Troubleshooting Common Issues:
Several challenges can arise during the synthesis of aspirin. Understanding and addressing them is crucial for a successful outcome:
-
Low Yield: Low yield can result from insufficient reaction time, inadequate mixing, inefficient heating, or loss of product during purification.
-
Impure Product: Impurities might arise from incomplete reaction, inadequate purification, or side reactions.
-
Difficult Recrystallization: Difficulty in obtaining high-quality crystals during recrystallization can be due to an inappropriate solvent choice, too rapid cooling, or insufficient purification before recrystallization.
Conclusion:
The reaction between salicylic acid and acetic anhydride is a fundamental example of an esterification reaction with significant practical importance. By understanding the reaction mechanism, optimizing reaction conditions, and employing appropriate purification techniques, it is possible to synthesize high-yield, high-purity aspirin. This synthesis demonstrates the power of organic chemistry to create valuable compounds from readily available starting materials. Ongoing research into greener and more sustainable methods ensures the continuous improvement of this classic reaction. The understanding of this reaction is not only important for aspiring chemists but also provides insight into the industrial production of a widely used drug. Further exploration of variations and improvements to this synthesis contributes to both academic understanding and practical applications in the pharmaceutical industry. The precise control of reaction parameters, careful monitoring of the process, and meticulous attention to purification are key elements in successfully synthesizing aspirin, solidifying its place as a quintessential example in organic chemistry education and industrial practice.
Latest Posts
Latest Posts
-
What Is The Molecular Mass Of Kno3
May 09, 2025
-
The Two Sides Of Dna Are Held Together By
May 09, 2025
-
Three Main Parts Of A Seed
May 09, 2025
-
Which Of The Following Is Strong Electrolyte
May 09, 2025
-
Why Do Skeletal Muscles Work In Pairs
May 09, 2025
Related Post
Thank you for visiting our website which covers about Reaction Of Salicylic Acid With Acetic Anhydride . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.