Protein Polymers Are Made Up Of
Juapaving
Mar 17, 2025 · 6 min read
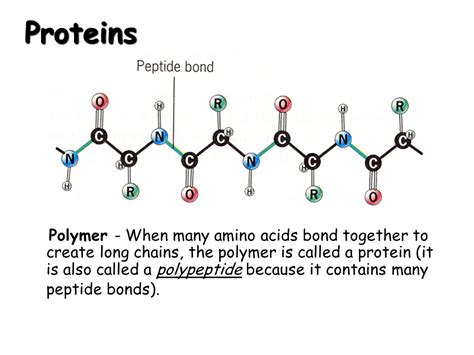
Table of Contents
Protein Polymers: A Deep Dive into Their Composition and Structure
Proteins are the workhorses of life, crucial for virtually every biological process. Understanding their structure is paramount to understanding their function. This comprehensive article explores the fundamental building blocks of protein polymers, delving into the intricate details of their composition and the diverse structures they form.
The Monomers of Protein Polymers: Amino Acids
Protein polymers, also known as polypeptides, are made up of smaller monomeric units called amino acids. These aren't just simple units; they are incredibly diverse molecules that dictate the final structure and function of the protein. There are 20 standard amino acids commonly found in proteins, each with unique characteristics that contribute to the overall protein properties.
The Structure of an Amino Acid
Each amino acid shares a common core structure consisting of:
- A central carbon atom (α-carbon): This carbon atom is bonded to four different groups.
- An amino group (-NH₂): This is a basic group, capable of accepting a proton (H⁺).
- A carboxyl group (-COOH): This is an acidic group, capable of donating a proton (H⁺).
- A hydrogen atom (-H): Simple, yet crucial to the overall geometry.
- A side chain (R-group): This is the variable part of the amino acid, and it's the R-group that distinguishes one amino acid from another. The R-group can be anything from a simple hydrogen atom (as in glycine) to a complex ring structure (as in tryptophan).
The Diversity of Amino Acid Side Chains
The diversity of R-groups is what makes amino acids, and hence proteins, so versatile. These side chains can be:
- Nonpolar (hydrophobic): These side chains are repelled by water and tend to cluster together in the interior of a protein. Examples include alanine, valine, leucine, isoleucine, methionine, phenylalanine, and tryptophan.
- Polar (hydrophilic): These side chains are attracted to water and often found on the surface of a protein, interacting with the aqueous environment. Examples include serine, threonine, cysteine, asparagine, glutamine, tyrosine.
- Charged (hydrophilic): These side chains carry a net positive or negative charge at physiological pH. Positively charged amino acids (basic) include lysine, arginine, and histidine. Negatively charged amino acids (acidic) include aspartic acid and glutamic acid.
The chemical properties of these side chains – their polarity, charge, size, and ability to form hydrogen bonds – heavily influence how the protein folds and interacts with other molecules.
Peptide Bonds: Linking Amino Acids Together
Amino acids are joined together by peptide bonds. This is a covalent bond formed between the carboxyl group (-COOH) of one amino acid and the amino group (-NH₂) of another amino acid. This reaction releases a molecule of water (H₂O), a process known as a dehydration reaction or condensation reaction.
The peptide bond itself has some unique characteristics:
- Partial double-bond character: The peptide bond exhibits resonance, meaning the electrons are delocalized, leading to a partially double-bond character. This restricts rotation around the peptide bond, influencing the overall protein conformation.
- Planar structure: Due to the partial double bond character, the six atoms involved in the peptide bond (C=O, N-H, and the α-carbons) lie in a single plane.
A chain of amino acids linked by peptide bonds is called a polypeptide. Proteins are essentially one or more polypeptide chains folded into a specific three-dimensional structure.
Levels of Protein Structure
The three-dimensional structure of a protein is crucial for its function. Protein structure is often described in terms of four levels of organization:
1. Primary Structure: The Amino Acid Sequence
The primary structure of a protein is simply the linear sequence of amino acids in the polypeptide chain. This sequence is determined by the genetic code and is dictated by the order of nucleotides in the corresponding DNA sequence. The primary structure is fundamental because it dictates all higher levels of protein structure. Even a single amino acid substitution can drastically alter the protein's function, as seen in sickle cell anemia.
2. Secondary Structure: Local Folding Patterns
The secondary structure refers to local folding patterns within the polypeptide chain. These are stabilized by hydrogen bonds between the carbonyl oxygen (C=O) of one amino acid and the amide hydrogen (N-H) of another amino acid. Common secondary structures include:
- α-helices: A right-handed coil stabilized by hydrogen bonds between every fourth amino acid.
- β-sheets: Extended polypeptide chains arranged side-by-side, stabilized by hydrogen bonds between adjacent strands. β-sheets can be parallel or antiparallel, depending on the direction of the strands.
- Turns and loops: These are short regions that connect α-helices and β-sheets, often found on the protein surface.
The specific arrangement of secondary structures within a protein depends on the primary sequence and the interactions between amino acid side chains.
3. Tertiary Structure: The 3D Arrangement of a Polypeptide
The tertiary structure refers to the overall three-dimensional arrangement of a single polypeptide chain. This is determined by interactions between the amino acid side chains, including:
- Hydrophobic interactions: Nonpolar side chains cluster together in the protein's interior, away from the aqueous environment.
- Hydrogen bonds: Polar side chains can form hydrogen bonds with each other or with water molecules.
- Ionic bonds (salt bridges): Charged side chains can form electrostatic interactions.
- Disulfide bonds: Covalent bonds formed between cysteine residues, creating strong cross-links within the protein.
The tertiary structure is essential for the protein's function, as it creates a specific three-dimensional shape that allows the protein to bind to other molecules or catalyze reactions.
4. Quaternary Structure: Multiple Polypeptide Chains
Some proteins consist of multiple polypeptide chains, each with its own tertiary structure. The arrangement of these individual subunits is called the quaternary structure. The subunits are held together by the same types of interactions that stabilize tertiary structure: hydrophobic interactions, hydrogen bonds, ionic bonds, and disulfide bonds. Examples of proteins with quaternary structure include hemoglobin and antibodies.
Factors Influencing Protein Structure
Several factors can influence the final structure and stability of a protein polymer:
- Temperature: High temperatures can disrupt the weak interactions that stabilize protein structure, leading to denaturation.
- pH: Changes in pH can alter the charge of amino acid side chains, affecting ionic interactions and potentially disrupting protein structure.
- Salinity: High salt concentrations can disrupt ionic interactions and hydrophobic interactions, leading to protein denaturation.
- Reducing agents: Reducing agents such as β-mercaptoethanol can break disulfide bonds, disrupting protein structure.
- Chaperone proteins: These proteins assist in the proper folding of other proteins, preventing aggregation and misfolding.
Protein Folding and Misfolding
The process of a polypeptide chain folding into its functional three-dimensional structure is known as protein folding. This is a complex process influenced by many factors, including the amino acid sequence, the cellular environment, and chaperone proteins. Improper protein folding can lead to the formation of amyloid fibrils, which are associated with various diseases, including Alzheimer's disease and Parkinson's disease.
Conclusion
Protein polymers are complex and fascinating molecules whose structure is intimately linked to their function. Understanding the composition of amino acids, the formation of peptide bonds, and the different levels of protein structure is essential for comprehending the vast array of biological processes in which proteins participate. From the simple sequence of amino acids to the intricate three-dimensional architecture, the protein polymer's journey from synthesis to functional conformation is a testament to the elegance and precision of biological systems. Further research continues to unravel the complexities of protein structure and function, paving the way for advancements in medicine, biotechnology, and materials science. The exploration of protein polymers remains a dynamic and rewarding area of scientific inquiry.
Latest Posts
Latest Posts
-
What Is The Square Root Of Sixteen
Mar 17, 2025
-
Multiples Of 5 Up To 100
Mar 17, 2025
-
What Is The Difference Between A Solute And A Solvent
Mar 17, 2025
-
How To Prove A Square Is A Square
Mar 17, 2025
-
Is 34 An Even Or Odd Number
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Protein Polymers Are Made Up Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
