Number Of Valence Electrons In P
Juapaving
Mar 16, 2025 · 5 min read
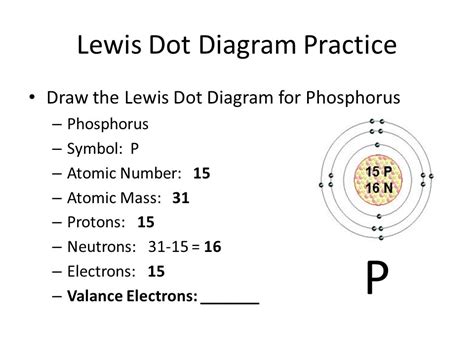
Table of Contents
Number of Valence Electrons in the p-Block Elements: A Comprehensive Guide
The periodic table is a powerful tool for understanding the behavior of elements. One crucial aspect of an element's properties is the number of valence electrons it possesses. These outermost electrons dictate how an atom interacts with other atoms, forming chemical bonds and influencing its reactivity. This article delves into the fascinating world of valence electrons, focusing specifically on the elements found in the p-block of the periodic table. We'll explore the trends, exceptions, and the significant implications of this electron configuration.
Understanding Valence Electrons
Before diving into the p-block, let's establish a firm understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell (also known as the valence shell) of an atom. These electrons are the ones most readily involved in chemical bonding. The number of valence electrons determines an element's chemical properties and its position in the periodic table. Elements in the same group (vertical column) have the same number of valence electrons, leading to similar chemical behaviors.
The p-Block: A Diverse Family of Elements
The p-block occupies a substantial portion of the periodic table, encompassing a wide array of elements with diverse properties. It's located to the right of the s-block and comprises Groups 13 to 18. The defining characteristic of p-block elements is that their valence electrons occupy the p orbitals. These orbitals can hold a maximum of six electrons. This explains the variation in valence electrons we observe in the p-block.
Determining the Number of Valence Electrons in p-Block Elements
The number of valence electrons for a p-block element can be readily determined using its group number. However, remember this rule applies only to the main group elements (those not in the transition metal series). The group number directly corresponds to the number of valence electrons:
- Group 13 (Boron Group): 3 valence electrons
- Group 14 (Carbon Group): 4 valence electrons
- Group 15 (Nitrogen Group): 5 valence electrons
- Group 16 (Oxygen Group or Chalcogens): 6 valence electrons
- Group 17 (Halogens): 7 valence electrons
- Group 18 (Noble Gases): 8 valence electrons (except for Helium, which has 2)
Example: Consider phosphorus (P), located in Group 15. It has 5 valence electrons. These electrons are distributed in the 3s and 3p orbitals – 2 in the 3s and 3 in the 3p.
Electron Configuration and Valence Electrons
Understanding electron configuration is key to comprehending valence electrons. Electron configuration describes how electrons are arranged in an atom's orbitals. For p-block elements, the general electron configuration is: ns<sup>2</sup> np<sup>x</sup>, where 'n' represents the principal quantum number (the energy level) and 'x' represents the number of electrons in the p orbitals (ranging from 1 to 6).
Let's analyze a few examples:
- Aluminum (Al): [Ne] 3s<sup>2</sup> 3p<sup>1</sup>. Aluminum has 3 valence electrons (2 from the 3s and 1 from the 3p).
- Silicon (Si): [Ne] 3s<sup>2</sup> 3p<sup>2</sup>. Silicon possesses 4 valence electrons.
- Chlorine (Cl): [Ne] 3s<sup>2</sup> 3p<sup>5</sup>. Chlorine has 7 valence electrons.
Trends in Valence Electron Number Across the p-Block
As we move across the p-block from left to right, the number of valence electrons systematically increases. This trend is a direct consequence of the filling of the p orbitals. Each successive element adds one more electron to the p subshells until the subshell is full with six electrons.
Exceptions and Anomalies
While the group number generally predicts the number of valence electrons, some exceptions exist. These exceptions are typically found in the transition metals and inner transition metals, where d and f orbitals participate in bonding. The involvement of d and f electrons complicates the straightforward relationship between group number and valence electrons.
Furthermore, the concept of "valence electrons" can become less straightforward for certain compounds and situations where electrons might be shared or delocalized across multiple atoms. Advanced concepts like oxidation states become more relevant in these cases.
Importance of Valence Electrons in Chemical Bonding
The number of valence electrons is paramount in determining an element's bonding behavior. Elements tend to react in ways that achieve a stable electron configuration, often resembling the noble gases (eight valence electrons, except for Helium with two). This is the foundation of the octet rule.
-
Ionic Bonding: Elements with few valence electrons (like alkali metals) tend to lose electrons to form positive ions (cations), while elements with many valence electrons (like halogens) gain electrons to form negative ions (anions). The electrostatic attraction between these oppositely charged ions forms an ionic bond.
-
Covalent Bonding: Elements with intermediate numbers of valence electrons often share electrons to achieve a stable octet, resulting in covalent bonds. This is common among non-metal elements in the p-block.
Applications and Relevance
Understanding the number of valence electrons in p-block elements is crucial in numerous fields:
-
Chemistry: Predicting the reactivity of elements, understanding chemical bonding, and designing new materials are all heavily reliant on knowing valence electron configurations.
-
Materials Science: The properties of semiconductors, insulators, and conductors are directly related to the number and arrangement of valence electrons in their constituent atoms. This knowledge is essential for developing advanced materials with specific electrical, optical, and mechanical properties.
-
Biological Sciences: The behavior of biomolecules like proteins and nucleic acids is heavily influenced by the interactions between their constituent atoms, which are governed by valence electron configurations.
Conclusion
The number of valence electrons in p-block elements is a fundamental concept in chemistry and related fields. This article provided a comprehensive overview of determining the number of valence electrons, understanding their arrangement in orbitals, and exploring the trends and exceptions across the p-block. The significance of valence electrons extends far beyond simply categorizing elements; it forms the basis for understanding chemical bonding, predicting reactivity, and designing new materials with tailored properties. By understanding this fundamental concept, we gain a deeper appreciation for the intricate relationship between electron configuration and the observable properties of matter. Further exploration into the specific properties and applications of individual p-block elements will deepen your understanding of this fascinating area of chemistry.
Latest Posts
Latest Posts
-
Ground State Electron Configuration For Pb
Mar 17, 2025
-
Power Is A Vector Or Scalar
Mar 17, 2025
-
What Is A Multiple Of 50
Mar 17, 2025
-
Least Common Denominator Of 2 And 8
Mar 17, 2025
-
How To Solve For Supplementary Angles
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In P . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
