Number Of Particles In One Mole Of Any Substance
Juapaving
Mar 19, 2025 · 5 min read
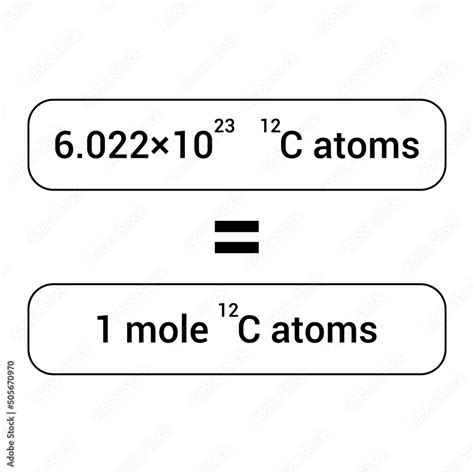
Table of Contents
Delving into the Avogadro Constant: The Number of Particles in a Mole
The concept of a mole is fundamental to chemistry, providing a bridge between the macroscopic world we observe and the microscopic world of atoms and molecules. Understanding the number of particles within a mole – a seemingly abstract concept – is crucial for accurate stoichiometric calculations, quantitative analysis, and a deeper comprehension of chemical reactions. This article will explore the Avogadro constant, its significance, and its implications in various chemical contexts.
What is a Mole?
A mole (mol) is the International System of Units (SI) base unit for the amount of substance. It's defined as exactly 6.02214076 × 10²³ elementary entities. These entities can be atoms, molecules, ions, electrons, or any other specified group of particles. Think of it like a convenient counting unit, much like a dozen (12) or a gross (144). Instead of counting individual atoms, which is practically impossible, we use the mole to represent a vast quantity.
The Avogadro Constant: A Cornerstone of Chemistry
The number 6.02214076 × 10²³ is known as the Avogadro constant (N<sub>A</sub>). This constant is not arbitrarily chosen; it's derived from the relationship between the atomic mass unit (amu) and the gram. One amu is defined as 1/12 the mass of a carbon-12 atom. The Avogadro constant is the number of atoms in exactly 12 grams of carbon-12.
This connection highlights the mole's practical utility. It provides a direct link between the microscopic atomic mass (in amu) and the macroscopic mass (in grams) of a substance. For instance, the atomic mass of oxygen is approximately 16 amu. This means that one mole of oxygen atoms has a mass of approximately 16 grams.
The Historical Context of Avogadro's Number
While the Avogadro constant is named after Amedeo Avogadro, he didn't actually determine its value. Avogadro proposed Avogadro's law (equal volumes of gases at the same temperature and pressure contain the same number of molecules), which laid the groundwork for determining this fundamental constant. Over time, scientists, using various experimental techniques, refined the value of this constant, culminating in its current precise definition.
Calculating the Number of Particles
Using the Avogadro constant is straightforward. To find the number of particles in a given number of moles, you simply multiply the number of moles by the Avogadro constant:
Number of particles = Number of moles × Avogadro constant (N<sub>A</sub>)
For example, to find the number of atoms in 2 moles of iron:
Number of atoms = 2 mol × 6.022 × 10²³ atoms/mol ≈ 1.204 × 10²⁴ atoms
This calculation applies equally to molecules. If you have 0.5 moles of water (H₂O), the number of water molecules would be:
Number of molecules = 0.5 mol × 6.022 × 10²³ molecules/mol ≈ 3.011 × 10²³ molecules
Beyond Atoms and Molecules: Extending the Concept
The mole's application extends beyond simple atoms and molecules. It can be used to quantify:
- Ions: The number of ions in a given amount of a salt solution.
- Formula Units: The number of formula units in an ionic compound (e.g., NaCl).
- Electrons: The number of electrons involved in a redox reaction.
- Photons: In photochemistry, the number of photons absorbed or emitted.
This versatility makes the mole an indispensable tool across various chemical disciplines.
The Mole and Molar Mass: Connecting Mass and Amount
The molar mass of a substance is the mass of one mole of that substance, usually expressed in grams per mole (g/mol). The molar mass is numerically equal to the average atomic mass (for elements) or molecular mass (for compounds) in amu.
This connection allows us to easily convert between mass and the number of moles:
Number of moles = Mass (g) / Molar mass (g/mol)
For instance, to find the number of moles in 10 grams of sodium chloride (NaCl), whose molar mass is approximately 58.44 g/mol:
Number of moles = 10 g / 58.44 g/mol ≈ 0.171 mol
Practical Applications of the Avogadro Constant
The Avogadro constant is not just a theoretical concept; it has numerous practical applications:
- Stoichiometry: Accurate stoichiometric calculations rely heavily on the mole concept to determine reactant ratios and product yields in chemical reactions.
- Titrations: In titrations, the mole concept is essential to determine the concentration of an unknown solution.
- Analytical Chemistry: Quantitative analysis techniques, such as gravimetric analysis and spectrophotometry, utilize the mole concept for accurate measurements.
- Industrial Chemistry: In industrial processes, precise control of reactant amounts often involves molar quantities to optimize production efficiency.
- Materials Science: The synthesis and characterization of materials often involve calculations based on molar quantities of different components.
Beyond Chemistry: Applications in Other Fields
The concept of a defined number of entities is not limited to chemistry. In physics, the Avogadro constant finds application in:
- Solid-State Physics: Determining the number of atoms in a crystal lattice.
- Nuclear Physics: Calculating the number of radioactive atoms in a sample.
The Precision and Future of the Avogadro Constant
The current definition of the Avogadro constant is incredibly precise. The ongoing refinements in measurement techniques continue to improve the accuracy. This precision is crucial for high-precision scientific work and ensures the reliability of numerous experimental findings.
Conclusion: A Universal Counting Unit
The Avogadro constant and the mole concept are cornerstones of chemistry and many related scientific fields. Understanding the precise number of particles in a mole provides a vital link between the microscopic and macroscopic worlds. Its application extends across various areas of science, highlighting its importance in quantitative analysis, stoichiometric calculations, and the deeper understanding of chemical phenomena. The ongoing refinement of the Avogadro constant underscores the continuous evolution of scientific measurement and its crucial role in advancing our understanding of the universe. From simple stoichiometric problems to complex industrial processes, the mole serves as a powerful and indispensable tool. Its significance in connecting the atomic world with everyday macroscopic observations makes it a fundamental concept for anyone exploring the fascinating realm of chemistry and its related disciplines.
Latest Posts
Latest Posts
-
What Is The Least Common Multiple Of 4 5 6
Mar 19, 2025
-
What Is The Lcm Of 2 3 And 4
Mar 19, 2025
-
How To Get A Percentage From A Ratio
Mar 19, 2025
-
How To Find Gcd Of 3 Numbers
Mar 19, 2025
-
Where Do Dark Reactions Take Place
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Number Of Particles In One Mole Of Any Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
