Name 3 Ways To Dissolve Something Faster.
Juapaving
Mar 04, 2025 · 6 min read
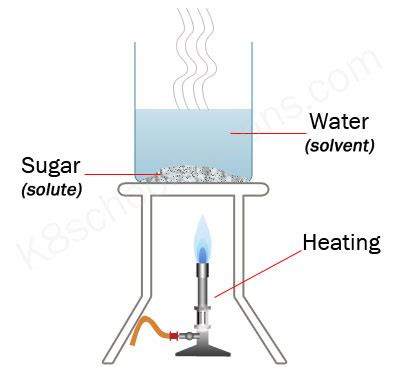
Table of Contents
3 Ways to Dissolve Something Faster: A Deep Dive into Dissolution Kinetics
Dissolution, the process where a solid, liquid, or gaseous substance dissolves into a solvent to form a solution, is a fundamental concept in chemistry and various industries. Understanding how to accelerate this process can be crucial in applications ranging from pharmaceutical drug delivery to industrial chemical processing. This article explores three key ways to significantly speed up the dissolution rate of a substance: increasing temperature, increasing surface area, and stirring or agitation. We'll delve into the scientific principles behind each method and explore practical examples of their application.
1. Increasing Temperature: Harnessing the Power of Kinetic Energy
Temperature plays a pivotal role in the dissolution process. Higher temperatures generally lead to faster dissolution rates. This is primarily due to the increased kinetic energy of both the solvent molecules and the solute particles.
The Role of Kinetic Energy
At higher temperatures, solvent molecules possess greater kinetic energy, allowing them to more effectively collide with and break apart the bonds holding the solute together. This increased collision frequency and energy translates directly to a higher dissolution rate. Imagine trying to break a pile of sugar cubes with your hands – the more forceful (higher energy) your hits, the faster the cubes will break apart and dissolve. This analogy mirrors the effect of temperature on solvent molecules.
Practical Applications and Examples
The impact of temperature on dissolution is widely observed in everyday life and across various industries:
- Dissolving Sugar in Hot Tea: Sugar dissolves significantly faster in hot tea than in iced tea. The increased kinetic energy of the hot water molecules facilitates a rapid breakdown of the sugar crystals.
- Pharmaceutical Drug Dissolution: Many pharmaceutical formulations rely on temperature control to ensure rapid drug dissolution and subsequent bioavailability. Warmer body temperatures contribute to faster absorption of orally administered medications.
- Industrial Chemical Processes: In industrial settings, controlled heating is often used to accelerate dissolution processes, improving efficiency and reducing processing times. For example, dissolving specific salts or reactants in chemical synthesis often requires heating to reach optimal reaction rates.
Limitations and Considerations
While increasing temperature is generally effective, it's important to consider the following:
- Solubility Limits: Increasing temperature doesn't always lead to unlimited solubility. For some substances, solubility increases with temperature, while for others, the effect may be minimal or even reversed. Understanding the solubility curve of the solute-solvent system is crucial.
- Thermal Degradation: Some substances are sensitive to heat and may degrade or decompose at higher temperatures, negatively impacting the overall process or producing unwanted byproducts. Carefully controlling the temperature is essential to prevent this.
- Energy Costs: Heating requires energy input, which can increase the overall cost of the dissolution process. Optimizing the temperature for maximum dissolution rate while minimizing energy consumption is a key consideration.
2. Increasing Surface Area: Maximizing Contact Points
The surface area of the solute significantly impacts dissolution rate. A larger surface area exposes more solute particles to the solvent, leading to increased interaction and faster dissolution.
The Principle of Surface Area
Imagine trying to dissolve a large sugar cube versus a spoonful of granulated sugar. The granulated sugar, with its significantly larger surface area, will dissolve much faster because more sugar particles are simultaneously exposed to the solvent. This increased contact between solvent and solute molecules accelerates the dissolution process.
Methods for Increasing Surface Area
Several techniques can be employed to increase the surface area of a solute:
- Particle Size Reduction: Grinding or milling a solid solute into smaller particles dramatically increases its surface area. This is a common technique used in pharmaceutical and food industries to improve dissolution.
- Using Powdered or Granulated Forms: Powdered or granulated forms of solutes naturally possess larger surface areas compared to solid blocks or crystals. This explains why powdered sugar dissolves much faster than a sugar cube.
- Creating Porous Structures: Materials with porous structures have larger effective surface areas due to their internal channels and voids. This principle is utilized in designing catalysts and adsorbents.
Practical Applications and Examples
The impact of surface area is evident in numerous applications:
- Dissolving Medications: Pharmaceutical tablets are often designed with specific formulations and coatings to control the rate of dissolution and drug release.
- Food Processing: The milling and grinding of ingredients in food processing are primarily aimed at increasing surface area for improved mixing, extraction, and dissolution. For example, finely ground coffee dissolves faster than coarsely ground coffee.
- Catalysis: Catalysts often have a high surface area to maximize the contact between the catalyst and the reactants, accelerating the reaction rate.
Limitations and Considerations
While increasing surface area is highly effective, there are limitations:
- Agglomeration: Fine particles sometimes agglomerate or clump together, reducing the effective surface area. Techniques to prevent agglomeration, such as the use of dispersing agents, may be necessary.
- Dust Formation: Generating fine particles can lead to dust formation, which can pose safety and environmental concerns. Appropriate handling and safety precautions are necessary.
- Cost of Particle Size Reduction: Reducing particle size requires energy input through milling or grinding, which can increase the overall cost.
3. Stirring or Agitation: Enhancing Mass Transfer
Stirring or agitating the solution significantly enhances the dissolution rate. This enhances mass transfer by continuously replenishing the solvent surrounding the solute particles.
The Principle of Mass Transfer
When a solute dissolves, a layer of concentrated solute forms around the dissolving particle. This layer hinders further dissolution by reducing the concentration gradient between the solute and the bulk solvent. Stirring or agitation breaks up this concentrated layer, continuously supplying fresh solvent to the solute particles, thereby maintaining a higher concentration gradient and accelerating dissolution.
Methods of Stirring and Agitation
Numerous methods can be employed to stir or agitate a solution:
- Manual Stirring: Simple manual stirring with a spoon or spatula can be effective for small-scale dissolution.
- Mechanical Stirring: Using a magnetic stirrer with a stir bar is a common method for laboratory and small-scale applications.
- Impellers and Agitators: For larger-scale industrial processes, impellers and agitators are used to create efficient mixing and mass transfer.
- Ultrasonication: Ultrasonic waves can create cavitation bubbles that enhance mass transfer and accelerate dissolution.
Practical Applications and Examples
The benefits of stirring are widely applied:
- Laboratory Chemistry: Magnetic stirrers are ubiquitous in chemistry laboratories to accelerate dissolution and reaction processes.
- Industrial Mixing: Large-scale industrial mixing tanks utilize powerful agitators to ensure homogeneous mixing and rapid dissolution of solids.
- Food and Beverage Industry: Mixing and stirring are critical in the food and beverage industry to ensure uniform distribution of ingredients and flavors.
Limitations and Considerations
While stirring is a powerful technique, considerations include:
- Shear Forces: High-speed stirring can generate shear forces that can damage sensitive materials or create emulsions.
- Energy Consumption: Agitation consumes energy, especially in large-scale processes. Optimizing the stirring speed and design is crucial for efficiency.
- Scale-up Challenges: Scaling up stirring processes from laboratory to industrial scales can present challenges in maintaining consistent mixing and mass transfer.
Conclusion: Optimizing Dissolution for Enhanced Efficiency
Accelerating dissolution is crucial in numerous applications. By strategically manipulating temperature, surface area, and agitation, we can significantly enhance the dissolution rate. Understanding the underlying scientific principles and carefully considering the limitations of each technique allows for the optimization of dissolution processes, leading to improved efficiency, reduced processing times, and enhanced product quality across various industries. Remember that the optimal approach often involves a combination of these methods tailored to the specific solute, solvent, and application.
Latest Posts
Latest Posts
-
Which Of The Following Statements Is Not True
Mar 04, 2025
-
What Is 2 3 As A Percent
Mar 04, 2025
-
How Many Feet Is 36 Inches
Mar 04, 2025
-
What Is The Major Product Of The Following Reaction
Mar 04, 2025
-
What Is The Square Root Of 50
Mar 04, 2025
Related Post
Thank you for visiting our website which covers about Name 3 Ways To Dissolve Something Faster. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
