Most Abundant Metal In Earth Crust
Juapaving
Mar 21, 2025 · 7 min read
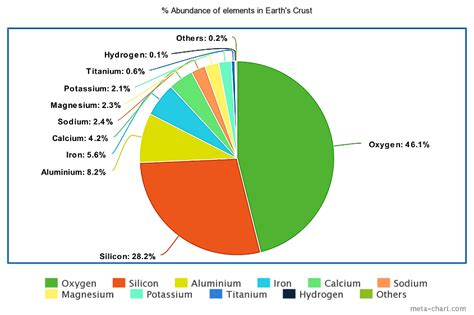
Table of Contents
The Most Abundant Metal in Earth's Crust: Aluminum's Reign and its Impact
Aluminum. The name might conjure images of sleek smartphones, lightweight airplanes, or recyclable soda cans. But beyond its modern applications, aluminum holds a far more significant position: it's the most abundant metal in Earth's crust. Understanding its prevalence, properties, and impact is crucial to comprehending our planet's geology and the materials that shape our world. This comprehensive guide delves deep into the fascinating world of aluminum, exploring its geological significance, extraction processes, and widespread applications.
Aluminum's Geological Abundance: A Deep Dive
While oxygen and silicon are the most abundant elements overall in the Earth's crust, aluminum reigns supreme among metals. Constituting approximately 8.1% of the Earth's crust by weight, it significantly surpasses iron (5%), calcium (3.6%), sodium (2.8%), potassium (2.6%), and magnesium (2.1%). This dominance isn't merely a numerical curiosity; it has profound implications for geology, material science, and even our daily lives.
Where to Find Aluminum: Minerals and Ore Deposits
Aluminum isn't found freely in nature due to its high reactivity. Instead, it exists primarily in compound form within various minerals. The most commercially important aluminum-containing mineral is bauxite, a sedimentary rock rich in hydrated aluminum oxides like gibbsite, boehmite, and diaspore. These minerals are the primary source for aluminum extraction.
Bauxite deposits are formed through intense weathering processes in tropical and subtropical regions. The process involves the leaching of silica and other elements from aluminum-rich parent rocks, leaving behind a residue concentrated in aluminum hydroxides. This explains why many significant bauxite reserves are located in regions with a history of intense weathering and tropical climates, including Australia, Guinea, Brazil, India, and Jamaica.
Other aluminum-containing minerals exist, but their economic viability for aluminum extraction is often limited due to lower aluminum content or the presence of other, less desirable elements. These include:
- Feldspars: A significant group of minerals making up a substantial portion of many igneous rocks. While containing aluminum, the extraction of aluminum from feldspars is currently not economically feasible on a large scale.
- Clay minerals: These are formed through the weathering of other aluminum-containing minerals and represent a vast reservoir of aluminum. However, the concentration of aluminum in clays is generally too low for efficient extraction.
- Micas: These silicate minerals contain aluminum but are predominantly used for other applications, rather than as an aluminum source.
The Geological History of Aluminum: A Story Etched in Rock
The abundance of aluminum in the Earth's crust is a testament to its formation and distribution throughout geological history. During the Earth's early formation, the heavier elements sank towards the core, while lighter elements, including aluminum, rose to the upper mantle and crust. Subsequent geological processes, such as volcanic activity, tectonic plate movement, and weathering, further shaped the distribution of aluminum-rich minerals.
The formation of bauxite deposits specifically highlights the interplay between chemical weathering, climate, and geological time. Millions of years of weathering under specific conditions are necessary to concentrate aluminum to economically viable levels. This intricate geological history emphasizes the intricate processes that have led to the current abundance of this vital metal.
Aluminum Extraction: From Bauxite to Ingots
Extracting aluminum from bauxite isn't a simple process. It requires a multi-stage operation involving significant energy input and sophisticated technology. The process, known as the Bayer process, is pivotal in transforming raw bauxite into pure aluminum oxide (alumina), which then undergoes electrolysis in the Hall-Héroult process to yield aluminum metal.
The Bayer Process: Purifying the Ore
The Bayer process begins by crushing and grinding bauxite ore. The crushed ore is then digested under pressure with a strong alkaline solution (sodium hydroxide) at high temperatures. This process dissolves the aluminum hydroxides in the bauxite, leaving behind impurities like iron oxides, silica, and titanium dioxide as a solid residue called red mud.
The aluminum-containing solution is then separated from the red mud, cooled, and seeded to precipitate aluminum hydroxide (Al(OH)₃). This precipitate is then calcined (heated) at high temperatures to produce alumina (Al₂O₃), a white powder that's the essential raw material for the next stage of aluminum production.
The Hall-Héroult Process: Electrolysis to the Rescue
Alumina is a stable compound and requires a significant amount of energy to break down into its constituent elements. The Hall-Héroult process uses electrolysis to achieve this. Molten alumina is dissolved in molten cryolite (Na₃AlF₆), a natural mineral that acts as a solvent and lowers the melting point of alumina. This molten mixture is then electrolyzed using a carbon anode and a carbon cathode.
During electrolysis, the aluminum ions (Al³⁺) are reduced at the cathode, forming liquid aluminum that collects at the bottom of the electrolytic cell. Oxygen ions (O²⁻) are oxidized at the anode, reacting with the carbon anode to produce carbon dioxide (CO₂) and carbon monoxide (CO). This process requires substantial electrical energy, typically around 13–15 kWh per kilogram of aluminum produced.
Aluminum's Versatility: A Multifaceted Metal
The unique properties of aluminum – its lightweight, high strength-to-weight ratio, corrosion resistance, ductility, and excellent conductivity – make it exceptionally versatile. This explains its dominance in numerous industries.
Transportation: Lighter Vehicles, Greater Efficiency
Aluminum plays a crucial role in the transportation industry. Its lightweight nature contributes to fuel efficiency in vehicles. Its use in automotive parts, aircraft bodies, and railway carriages is ever-increasing. The trend toward lighter and more fuel-efficient vehicles further fuels the demand for aluminum.
Packaging: The Unsung Hero of Everyday Life
Aluminum's corrosion resistance and recyclability make it a preferred material for packaging. From cans to foil to food containers, aluminum protects products from degradation and spoilage. The widespread use of aluminum cans, in particular, highlights its environmental benefits as it is readily recyclable.
Construction: Building a Sustainable Future
Aluminum's strength, durability, and weather resistance make it a valuable material in construction. Its use in building facades, roofing, window frames, and structural components is steadily growing. Its lightweight yet strong nature makes it suitable for constructing long-span structures with minimal weight.
Electrical Applications: Powering Modern Technology
Aluminum's excellent electrical conductivity positions it as a vital material in electrical applications. It's used in power transmission lines, electrical wiring, and components in electronic devices. The efficiency of its conductivity makes it a cost-effective choice for large-scale power distribution.
Other Applications: A Diverse Range of Uses
Beyond these major applications, aluminum finds use in a wide array of other industries:
- Aerospace: Its lightweight strength is essential for aircraft manufacturing.
- Medical: Used in surgical instruments, implants, and prosthetics.
- Consumer Goods: Utilized in kitchen utensils, appliances, and sporting goods.
- Defense: Featured in military vehicles, weaponry, and equipment.
The Environmental Impact of Aluminum: Balancing Progress and Sustainability
While aluminum is a crucial material for modern society, its production and use have environmental implications. The Bayer process, in particular, generates significant amounts of red mud, a waste product containing heavy metals and other potentially harmful substances. Proper management and disposal of red mud are crucial to minimize environmental risks.
The energy-intensive nature of aluminum production also contributes to greenhouse gas emissions. However, advancements in technology, such as improved electrolytic processes and increased use of renewable energy sources, are aimed at reducing the environmental footprint of aluminum production.
The recyclability of aluminum is a significant advantage. Recycling aluminum requires significantly less energy compared to primary aluminum production, reducing energy consumption and greenhouse gas emissions. Promoting the recycling of aluminum is crucial for its sustainable utilization.
Conclusion: Aluminum's Enduring Importance
Aluminum's abundance in the Earth's crust, coupled with its remarkable properties and diverse applications, solidifies its position as a cornerstone of modern civilization. Understanding its geological significance, extraction processes, and environmental impact is crucial for responsible resource management and sustainable development. With continued innovation in extraction technologies and recycling practices, aluminum's role in shaping our future is guaranteed to be both significant and sustainable. The ongoing quest to optimize its production and minimize its environmental impact underscores its enduring importance in a world increasingly reliant on efficient and eco-conscious materials.
Latest Posts
Latest Posts
-
Whats The Difference Between Cell Wall And Cell Membrane
Mar 28, 2025
-
Why Are Leaves Green In Colour
Mar 28, 2025
-
What Type Of Mixture Is Concrete
Mar 28, 2025
-
Five Letter Word Ending In E R
Mar 28, 2025
-
The Largest Endocrine Gland Is The
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Most Abundant Metal In Earth Crust . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
