Molar Mass Of Mg3 Po4 2
Juapaving
May 12, 2025 · 5 min read
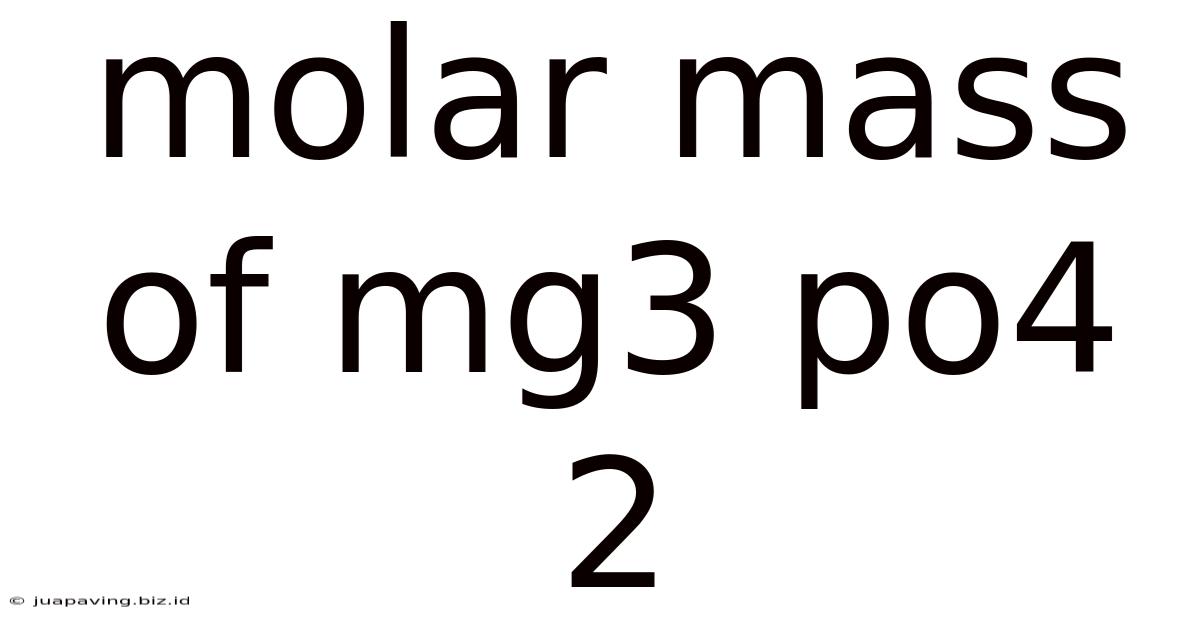
Table of Contents
Determining the Molar Mass of Mg₃(PO₄)₂: A Comprehensive Guide
The molar mass of a compound represents the mass of one mole of that substance. A mole, in chemistry, is a fundamental unit representing Avogadro's number (approximately 6.022 x 10²³) of particles, whether atoms, molecules, ions, or formula units. Calculating the molar mass is crucial in various stoichiometric calculations, such as determining reactant amounts in chemical reactions or analyzing the composition of compounds. This article provides a detailed explanation of how to determine the molar mass of magnesium phosphate, Mg₃(PO₄)₂, and explores its significance in chemistry.
Understanding the Chemical Formula: Mg₃(PO₄)₂
Before calculating the molar mass, let's decipher the chemical formula, Mg₃(PO₄)₂. This formula tells us that one formula unit of magnesium phosphate consists of:
- Three (3) magnesium (Mg) atoms: Magnesium is an alkaline earth metal, a reactive element crucial in numerous biological processes.
- Two (2) phosphate (PO₄) ions: Phosphate is a polyatomic ion, meaning it's a group of atoms carrying a net charge. Each phosphate ion consists of one phosphorus (P) atom and four oxygen (O) atoms, carrying a -3 charge (PO₄³⁻).
The subscript numbers indicate the number of atoms or ions present in one formula unit. The parentheses around (PO₄) indicate that the entire phosphate ion is multiplied by the subscript 2.
Step-by-Step Calculation of the Molar Mass of Mg₃(PO₄)₂
Calculating the molar mass involves summing the atomic masses of all the atoms present in the formula unit. We'll use the standard atomic weights from the periodic table:
- Magnesium (Mg): Approximately 24.31 g/mol
- Phosphorus (P): Approximately 30.97 g/mol
- Oxygen (O): Approximately 16.00 g/mol
1. Calculate the mass of magnesium atoms:
- 3 Mg atoms × 24.31 g/mol/Mg atom = 72.93 g/mol
2. Calculate the mass of phosphorus atoms:
- 2 P atoms × 30.97 g/mol/P atom = 61.94 g/mol
3. Calculate the mass of oxygen atoms:
- Each phosphate ion (PO₄) contains 4 oxygen atoms, and there are 2 phosphate ions. Therefore, there are a total of 8 oxygen atoms.
- 8 O atoms × 16.00 g/mol/O atom = 128.00 g/mol
4. Sum the masses of all atoms:
- Total molar mass = Mass of Mg + Mass of P + Mass of O
- Total molar mass = 72.93 g/mol + 61.94 g/mol + 128.00 g/mol = 262.87 g/mol
Therefore, the molar mass of Mg₃(PO₄)₂ is approximately 262.87 grams per mole.
Significance of Molar Mass in Chemical Calculations
The molar mass of Mg₃(PO₄)₂ is crucial in a wide range of chemical calculations, including:
1. Stoichiometry:
Stoichiometry involves calculating the quantities of reactants and products in chemical reactions. Knowing the molar mass of Mg₃(PO₄)₂ allows us to convert between mass and moles of the compound. For instance, if we know the mass of magnesium phosphate used in a reaction, we can determine the number of moles using the following formula:
- Moles = Mass (g) / Molar Mass (g/mol)
Conversely, if we know the number of moles, we can calculate the mass.
2. Determining Empirical and Molecular Formulas:
Molar mass is essential in determining the empirical and molecular formulas of compounds. The empirical formula represents the simplest whole-number ratio of atoms in a compound. The molecular formula represents the actual number of atoms in a molecule. Knowing the molar mass helps to relate the empirical formula to the molecular formula.
3. Solution Chemistry:
Molar mass is crucial in preparing solutions of known concentrations. For instance, to prepare a 1M (one molar) solution of Mg₃(PO₄)₂, we would need to dissolve 262.87 grams of Mg₃(PO₄)₂ in enough solvent to make one liter of solution. The molar mass ensures the accurate preparation of solutions with precise concentrations.
4. Gravimetric Analysis:
Gravimetric analysis is a quantitative method used to determine the amount of a substance by measuring its mass. Knowing the molar mass of Mg₃(PO₄)₂ is essential in gravimetric analysis involving this compound, allowing conversion between mass and moles.
Applications of Magnesium Phosphate
Magnesium phosphate, Mg₃(PO₄)₂, finds numerous applications across various fields:
-
Agriculture: It serves as a valuable fertilizer, providing essential nutrients like magnesium and phosphorus to plants. These nutrients are crucial for plant growth and development, particularly in crops that exhibit magnesium or phosphorus deficiency. Understanding its molar mass is crucial in formulating balanced fertilizers.
-
Medicine: Magnesium phosphate is used in some medications as a laxative. Its ability to draw water into the intestines promotes bowel movements. Precise dosing, relying on molar mass calculations, ensures safe and effective use.
-
Food Industry: It can be used as a food additive, often serving as a leavening agent or a source of minerals. Its presence contributes to the overall nutritional profile of processed food products.
-
Industry: Magnesium phosphate is applied as a flame retardant in certain materials, enhancing fire safety and mitigating the risks associated with combustion. Its properties are exploited to improve the fire resistance of various products.
-
Environmental Science: Understanding its chemical properties, including molar mass, is relevant in various environmental studies, such as determining its solubility and potential impact on aquatic ecosystems. This helps assess its role in environmental processes and its contribution to water quality.
Conclusion
The molar mass of Mg₃(PO₄)₂, approximately 262.87 g/mol, is a fundamental value with widespread applications in various chemical calculations. Its accurate determination is critical for stoichiometric calculations, solution preparation, gravimetric analysis, and understanding the compound's role in diverse fields, ranging from agriculture to medicine and industrial applications. The step-by-step calculation method outlined in this article provides a clear and comprehensive approach to determine the molar mass of any compound. Understanding molar mass calculations is a cornerstone skill for anyone pursuing a career in chemistry or related scientific fields. Mastering this concept empowers one to perform accurate calculations, interpret data effectively, and contribute to the advancement of scientific knowledge. Furthermore, understanding the applications of Mg₃(PO₄)₂ illustrates the practical significance of chemical calculations in real-world applications.
Latest Posts
Latest Posts
-
Circle Has How Many Sides And Corners
May 13, 2025
-
Where Are Atp Synthase Complexes Located In Plant Cells
May 13, 2025
-
Which Is Larger 1 4 Or 3 16
May 13, 2025
-
How Many Meters Is 20 Cm
May 13, 2025
-
Resonance Structures Practice Problems With Answers
May 13, 2025
Related Post
Thank you for visiting our website which covers about Molar Mass Of Mg3 Po4 2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.