Molar Mass Of K2 Cr2 O7
Juapaving
May 14, 2025 · 5 min read
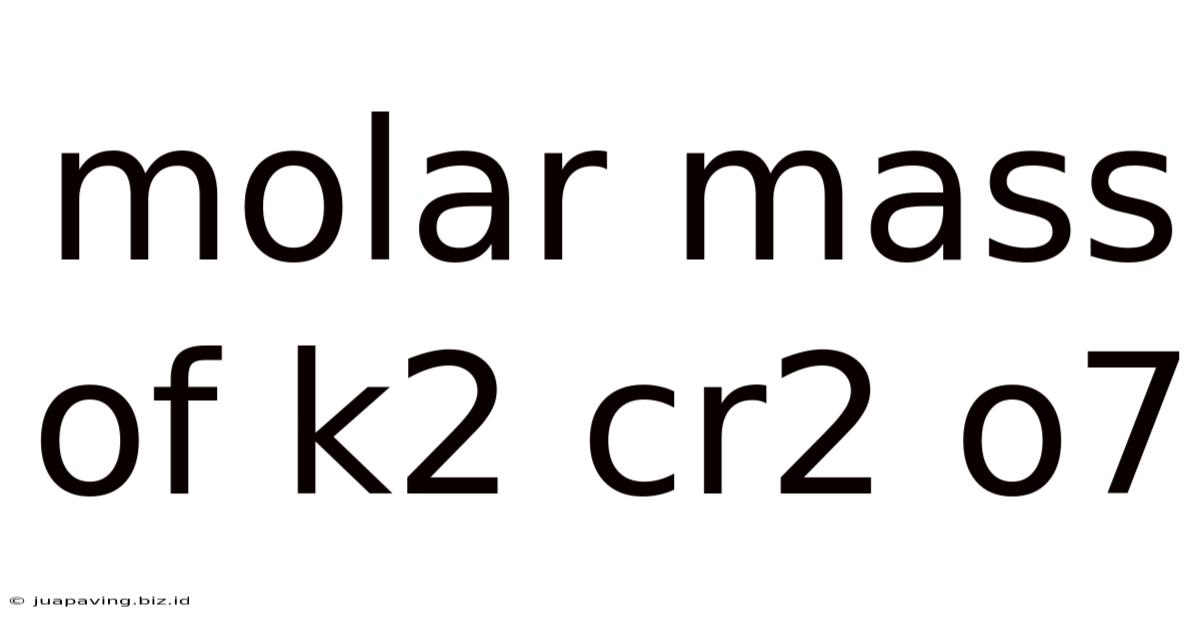
Table of Contents
Unveiling the Molar Mass of K₂Cr₂O₇: A Deep Dive into Potassium Dichromate
Potassium dichromate (K₂Cr₂O₇) is a vibrant orange, crystalline compound with a wide array of applications, from industrial uses like leather tanning and wood preservation to its role as a powerful oxidizing agent in various chemical reactions. Understanding its molar mass is crucial for accurate stoichiometric calculations and quantitative analysis in chemistry. This article will delve deep into the concept of molar mass, specifically focusing on K₂Cr₂O₇, explaining its calculation, significance, and applications.
What is Molar Mass?
The molar mass of a substance is defined as the mass of one mole of that substance. A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of constituent particles, whether they are atoms, molecules, ions, or other specified entities. The molar mass is expressed in grams per mole (g/mol). It essentially provides a bridge between the microscopic world of atoms and molecules and the macroscopic world of grams and kilograms that we measure in the laboratory.
Calculating Molar Mass: A Step-by-Step Guide
Calculating the molar mass of any compound, including K₂Cr₂O₇, involves adding up the atomic masses of all the atoms present in its chemical formula. We need to know the atomic masses of each element involved:
- Potassium (K): Approximately 39.10 g/mol
- Chromium (Cr): Approximately 51.99 g/mol
- Oxygen (O): Approximately 16.00 g/mol
Let's break down the calculation for K₂Cr₂O₇:
-
Identify the elements and their respective number of atoms: The formula K₂Cr₂O₇ shows that we have 2 potassium atoms, 2 chromium atoms, and 7 oxygen atoms.
-
Multiply the atomic mass of each element by its number of atoms:
- Potassium: 2 atoms * 39.10 g/mol/atom = 78.20 g/mol
- Chromium: 2 atoms * 51.99 g/mol/atom = 103.98 g/mol
- Oxygen: 7 atoms * 16.00 g/mol/atom = 112.00 g/mol
-
Sum up the individual masses: Adding the masses calculated in step 2 gives us the molar mass of K₂Cr₂O₇:
78.20 g/mol + 103.98 g/mol + 112.00 g/mol = 294.18 g/mol
Therefore, the molar mass of potassium dichromate (K₂Cr₂O₇) is approximately 294.18 g/mol.
The Significance of Molar Mass in Chemical Calculations
The molar mass of K₂Cr₂O₇, and any compound for that matter, is a cornerstone in various chemical calculations:
-
Stoichiometry: In stoichiometric calculations, molar mass is used to convert between the mass of a substance and the number of moles. This is vital for determining reactant quantities, product yields, and limiting reagents in chemical reactions. For example, if you know the mass of K₂Cr₂O₇ used in a reaction, you can use its molar mass to calculate the number of moles involved, enabling you to calculate the moles and mass of other reactants or products.
-
Solution Chemistry: Molar mass is essential for preparing solutions of known concentrations. For instance, to prepare a 1 M solution of K₂Cr₂O₇, you would need to dissolve 294.18 g of K₂Cr₂O₇ in enough solvent to make 1 liter of solution.
-
Titrations: In titrations, where one solution is added to another to reach a specific endpoint, the molar mass of K₂Cr₂O₇ is crucial for determining the concentration of an unknown solution.
-
Gas Laws: While not directly applicable in all gas law calculations, molar mass helps in determining the density of a gaseous substance, which is relevant in some gas law problems.
-
Quantitative Analysis: Molar mass is an integral part of quantitative analysis techniques like gravimetric analysis, where the mass of a precipitate is used to determine the amount of analyte in a sample.
Applications of Potassium Dichromate (K₂Cr₂O₇)
The applications of K₂Cr₂O₇ are extensive and span various fields:
-
Industrial Applications:
- Leather Tanning: K₂Cr₂O₇ is a crucial component in the tanning of leather, helping to convert animal hides into durable leather products.
- Wood Preservation: It acts as a wood preservative, protecting wood from rot and decay.
- Textile Industry: It finds use in the dyeing and printing of textiles.
- Photography: Historically, it was used in certain photographic processes.
-
Laboratory Applications:
- Oxidizing Agent: K₂Cr₂O₇ is a strong oxidizing agent and is used in numerous redox reactions in organic and inorganic chemistry. It's often used in titrations to determine the concentration of reducing agents.
- Cleaning Agent: In laboratory settings, it is sometimes used as a cleaning agent for glassware.
- Analytical Chemistry: It has applications in various analytical techniques.
-
Other Applications:
- Etching: Used in etching metals.
- Pigments: It contributes to the orange color in some pigments.
Safety Precautions When Handling K₂Cr₂O₇
It is imperative to emphasize that potassium dichromate is a toxic and harmful substance. It's a known carcinogen and can cause severe health problems through skin contact, inhalation, or ingestion. Always handle K₂Cr₂O₇ with utmost care and follow these safety precautions:
- Wear appropriate personal protective equipment (PPE): This includes gloves, eye protection, and a lab coat.
- Work in a well-ventilated area: To minimize inhalation hazards.
- Avoid skin contact: Wash thoroughly if skin contact occurs.
- Proper disposal: Dispose of K₂Cr₂O₇ waste according to local regulations.
- Consult Safety Data Sheets (SDS): Always refer to the SDS for detailed information on handling, storage, and disposal.
Conclusion: Molar Mass – A Key to Understanding K₂Cr₂O₇
The molar mass of K₂Cr₂O₇, at approximately 294.18 g/mol, is not merely a calculated value; it's a fundamental piece of information essential for understanding and utilizing this important compound. Its applications across various industries and scientific fields highlight its significance. However, it is crucial to remember the inherent dangers associated with handling K₂Cr₂O₇ and to always prioritize safety. By understanding its molar mass and employing proper safety protocols, we can harness the capabilities of this compound responsibly and effectively. Further research into its diverse applications continues to reveal its importance in various scientific and industrial processes. The precise calculation and application of its molar mass remain crucial for accurate and safe usage. Always prioritize safety when working with this potent chemical.
Latest Posts
Latest Posts
-
Number In Words From 1 To 100
May 14, 2025
-
What Is 96 Inches In Feet
May 14, 2025
-
What Percentage Is 35 Out Of 40
May 14, 2025
-
Electricity Is Measured In What Unit
May 14, 2025
-
Is A Pencil A Conductor Or Insulator
May 14, 2025
Related Post
Thank you for visiting our website which covers about Molar Mass Of K2 Cr2 O7 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.