Molar Mass Of Cuso4 X 5h2o
Juapaving
May 09, 2025 · 5 min read
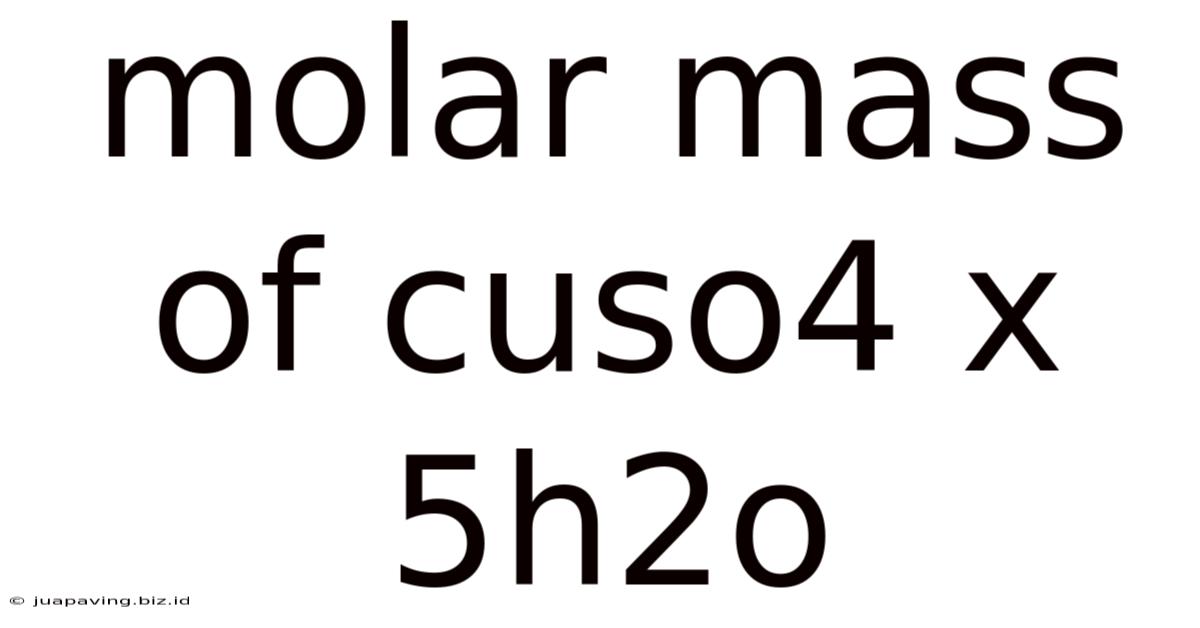
Table of Contents
Understanding the Molar Mass of CuSO₄·5H₂O: A Comprehensive Guide
Copper(II) sulfate pentahydrate, also known as CuSO₄·5H₂O, is a vibrant blue crystalline compound commonly used in various applications, from agricultural fertilizers to electroplating solutions. A crucial aspect of understanding and working with this chemical is grasping its molar mass. This article will delve deep into the calculation and significance of the molar mass of CuSO₄·5H₂O, exploring its applications and related concepts.
What is Molar Mass?
Before we calculate the molar mass of CuSO₄·5H₂O, let's define the term. Molar mass is the mass of one mole of a substance. A mole, according to Avogadro's number, contains approximately 6.022 x 10²³ particles (atoms, molecules, ions, etc.). Therefore, molar mass essentially tells us the mass of 6.022 x 10²³ particles of a given substance in grams. It's a fundamental concept in chemistry used in stoichiometric calculations, determining the concentration of solutions, and numerous other applications. The units for molar mass are grams per mole (g/mol).
Calculating the Molar Mass of CuSO₄·5H₂O
The formula CuSO₄·5H₂O indicates that one molecule of copper(II) sulfate pentahydrate consists of one copper(II) ion (Cu²⁺), one sulfate ion (SO₄²⁻), and five water molecules (5H₂O). To calculate its molar mass, we need the atomic masses of each element present:
- Copper (Cu): Approximately 63.55 g/mol
- Sulfur (S): Approximately 32.07 g/mol
- Oxygen (O): Approximately 16.00 g/mol
- Hydrogen (H): Approximately 1.01 g/mol
Now, let's break down the calculation:
- Cu: 1 Cu atom x 63.55 g/mol = 63.55 g/mol
- S: 1 S atom x 32.07 g/mol = 32.07 g/mol
- O (in CuSO₄): 4 O atoms x 16.00 g/mol = 64.00 g/mol
- O (in 5H₂O): 5 x (1 O atom x 16.00 g/mol) = 80.00 g/mol
- H (in 5H₂O): 5 x (2 H atoms x 1.01 g/mol) = 10.10 g/mol
Adding these values together gives us the total molar mass:
63.55 g/mol + 32.07 g/mol + 64.00 g/mol + 80.00 g/mol + 10.10 g/mol = 249.72 g/mol
Therefore, the molar mass of CuSO₄·5H₂O is approximately 249.72 g/mol. Slight variations might occur depending on the atomic mass values used, but this is a highly accurate approximation.
Significance of Molar Mass in CuSO₄·5H₂O Applications
Understanding the molar mass of CuSO₄·5H₂O is crucial for various applications:
1. Preparing Solutions of Specific Concentrations:
Many applications require preparing solutions of CuSO₄·5H₂O with precise concentrations (e.g., molarity). Knowing the molar mass allows accurate calculation of the mass required to prepare a solution of a desired molarity and volume. For instance, if you need to prepare 1 liter of a 0.1 M CuSO₄·5H₂O solution, you can use the following formula:
Moles = Molarity x Volume (in liters)
Mass = Moles x Molar Mass
By substituting the values, you can calculate the mass of CuSO₄·5H₂O required.
2. Stoichiometric Calculations:
In chemical reactions involving CuSO₄·5H₂O, the molar mass is essential for performing stoichiometric calculations. This involves determining the amounts of reactants and products involved in a chemical reaction based on their molar ratios.
3. Determining the Purity of CuSO₄·5H₂O Samples:
The molar mass can be used in analytical chemistry to determine the purity of a sample of CuSO₄·5H₂O. This often involves techniques like titration or gravimetric analysis, where the mass of the compound is related to its molar mass to calculate its purity.
4. Agricultural Applications:
In agriculture, copper sulfate is used as a micronutrient for plants. Accurate calculations using the molar mass are necessary to determine the amount needed for effective fertilization without causing toxicity.
Hydration and Anhydrous CuSO₄
It's important to note the significance of the "·5H₂O" in the formula. This indicates that five water molecules are bound to each copper sulfate molecule. This water is referred to as water of crystallization or water of hydration. Heating CuSO₄·5H₂O drives off this water, resulting in anhydrous copper(II) sulfate (CuSO₄), which is a white powder. The molar mass of anhydrous CuSO₄ is significantly lower than the pentahydrate form because it lacks the mass of the five water molecules. Calculating the molar mass of anhydrous CuSO₄ involves only the atomic masses of copper, sulfur, and oxygen.
The change in color from blue to white upon dehydration is a striking visual demonstration of the loss of water molecules. This property can be utilized for various applications, including water detection in solvents.
Related Concepts and Further Exploration
Understanding the molar mass of CuSO₄·5H₂O opens the door to exploring several related concepts in chemistry:
- Empirical Formula: Determining the simplest whole-number ratio of atoms in a compound.
- Molecular Formula: Determining the exact number of atoms of each element in a molecule.
- Percentage Composition: Calculating the percentage by mass of each element in a compound.
- Stoichiometry: The quantitative relationship between reactants and products in a chemical reaction.
- Solution Chemistry: Preparing and analyzing solutions of different concentrations.
Conclusion
The molar mass of CuSO₄·5H₂O, approximately 249.72 g/mol, is a fundamental piece of information for anyone working with this compound. Its accurate determination and application are essential for preparing solutions, performing stoichiometric calculations, determining purity, and understanding its various applications in diverse fields. By understanding the concept of molar mass and its calculation, one gains a deeper appreciation for the quantitative nature of chemistry and its impact on practical applications. This knowledge is a cornerstone for further exploration of chemical principles and analytical techniques. Further research into related concepts will enhance your understanding of chemical processes and applications involving copper sulfate pentahydrate.
Latest Posts
Latest Posts
-
Which Of The Following Is A Colligative Property
May 11, 2025
-
Relation Between Degree Fahrenheit And Degree Celsius
May 11, 2025
-
How Many Rows In Periodic Table
May 11, 2025
-
Is Chloride A Metal Or Nonmetal
May 11, 2025
-
A Substance That Dissolves In A Solvent
May 11, 2025
Related Post
Thank you for visiting our website which covers about Molar Mass Of Cuso4 X 5h2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.