Metals Are Located Where On The Periodic Table
Juapaving
Mar 06, 2025 · 6 min read
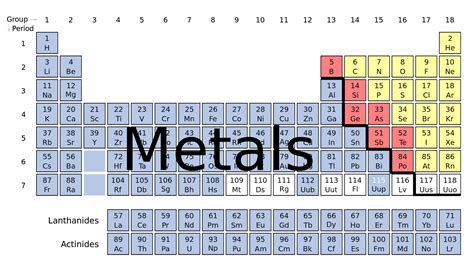
Table of Contents
Metals: Location and Properties on the Periodic Table
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and resulting properties. One of the most fundamental classifications of elements is the division into metals, nonmetals, and metalloids. Understanding where metals are located on the periodic table is crucial to understanding their chemical behavior and practical applications. This comprehensive guide delves into the precise location of metals, exploring their characteristic properties and providing examples of specific metal groups.
The Broad Location of Metals on the Periodic Table
Metals overwhelmingly dominate the periodic table. They occupy the left side and center of the table, forming a large, roughly stair-step shaped block. This block excludes the elements bordering a diagonal line running from boron (B) to astatine (At). Elements to the left of this line are generally considered metals, while those to the right are nonmetals. Elements directly on the line exhibit properties of both metals and nonmetals, and are classified as metalloids or semimetals.
The Stair-Step Line and Metalloids
The crucial "stair-step" line separating metals and nonmetals runs approximately from boron (B) to polonium (Po). This line isn't a rigid boundary; the properties of elements near this line are often transitional, meaning they display characteristics of both metals and nonmetals. These elements are called metalloids or semimetals, and their properties can vary greatly depending on external factors like temperature and pressure. Examples include silicon (Si), germanium (Ge), arsenic (As), and antimony (Sb).
Specific Metal Groups and Their Locations
Within the broad classification of metals, there's a further division into specific groups, each with unique properties and locations on the periodic table:
1. Alkali Metals (Group 1): The Most Reactive Metals
The alkali metals are located in Group 1 of the periodic table, the first column to the far left. This group includes lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). They are extremely reactive, readily losing one electron to form a +1 ion. Their reactivity increases as you move down the group. This high reactivity makes them useful in various applications, but also necessitates careful handling due to their tendency to react violently with water and air.
2. Alkaline Earth Metals (Group 2): Reactive, but Less So Than Alkali Metals
Found in Group 2, the alkaline earth metals—beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra)—are also reactive metals, but less so than the alkali metals. They typically lose two electrons to form a +2 ion. Although less reactive than Group 1 metals, they still require careful handling. These metals find use in various alloys and compounds. Magnesium, for example, is lightweight and strong, making it ideal for aerospace applications.
3. Transition Metals (Groups 3-12): The Diverse Metals
The transition metals occupy the central block of the periodic table (Groups 3-12). This is a large and diverse group of metals, characterized by their ability to form multiple oxidation states (different ionic charges). This versatility leads to a wide range of chemical and physical properties, making them crucial in countless industrial applications. Examples include iron (Fe), copper (Cu), gold (Au), and platinum (Pt). Their varied properties stem from the filling of the d-orbital electrons, leading to complex chemical behaviors and colorful compounds.
Specific Transition Metal Characteristics:
- Variable Oxidation States: This allows them to form a variety of compounds with different properties.
- Catalysis: Many transition metals are excellent catalysts, accelerating chemical reactions.
- Alloy Formation: They readily form alloys with other metals, creating materials with enhanced properties.
- Magnetic Properties: Some transition metals and their compounds exhibit strong magnetic properties (e.g., iron, nickel, cobalt).
4. Post-Transition Metals (Groups 13-15, somewhat overlapping): A Bridge Between Metals and Metalloids
These elements show a blending of metallic and non-metallic characteristics, lying between the transition metals and the metalloids. Their properties vary more significantly across the period than down the group. Examples include aluminum (Al), tin (Sn), and lead (Pb). They often exhibit lower melting points and less pronounced metallic properties compared to the transition metals.
5. Lanthanides and Actinides: The Inner Transition Metals
The lanthanides and actinides are located at the bottom of the periodic table, generally separated for space considerations. They are known as the inner transition metals, filling the f-orbitals. The lanthanides (rare earth elements) are characterized by their similar chemical properties, making separation challenging. Actinides are radioactive, with many being synthetically produced.
6. Other Metals Beyond the Main Groups
Beyond the previously mentioned groups, several other elements exhibit metallic characteristics and are considered metals, although they don't neatly fit into a specific group. This includes elements like polonium (Po) which, despite its proximity to the metalloid line, generally shows metallic properties at standard conditions.
Properties of Metals: A Summary
The location of metals on the periodic table directly relates to their characteristic properties:
- Good Electrical Conductivity: Metals are excellent conductors of electricity due to the presence of delocalized electrons.
- Good Thermal Conductivity: They efficiently transfer heat.
- Malleability: They can be hammered into sheets.
- Ductility: They can be drawn into wires.
- Luster: They have a shiny appearance.
- High Density: Generally denser than nonmetals.
- High Melting and Boiling Points: Many metals have high melting and boiling points. (exceptions exist)
- Positive Ions: They tend to lose electrons to form positive ions (cations).
Applications of Metals
The diverse properties of metals lead to their widespread use in various applications:
- Construction: Steel, aluminum, and other metals are fundamental in building construction.
- Transportation: Metals like steel and aluminum are used extensively in vehicles, airplanes, and ships.
- Electronics: Copper, gold, and other metals are essential in electronic devices.
- Medicine: Certain metals are used in medical implants and devices.
- Energy: Metals are crucial in energy production and storage technologies.
- Catalysis: Transition metals play vital roles as catalysts in various industrial processes.
Conclusion: Understanding the Periodic Table's Metal Map
The periodic table provides a powerful visual representation of the organization of elements and their properties. The location of metals, spread across the left and center of the table, reveals their chemical behavior and practical applications. Understanding the specific metal groups (alkali metals, alkaline earth metals, transition metals, etc.) and their distinct properties is essential for anyone studying chemistry or working in related fields. The diverse properties of metals, stemming from their electronic configurations, lead to their indispensable role in modern society. From construction and transportation to electronics and medicine, metals continue to play a critical and evolving role in shaping our world. Further exploration into specific metal groups and their applications will reveal even more fascinating aspects of the periodic table's intricate organization and the vital contributions of metals to our technological advancements.
Latest Posts
Related Post
Thank you for visiting our website which covers about Metals Are Located Where On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
