Melting Point Temperature In Celsius For Water
Juapaving
Mar 04, 2025 · 6 min read
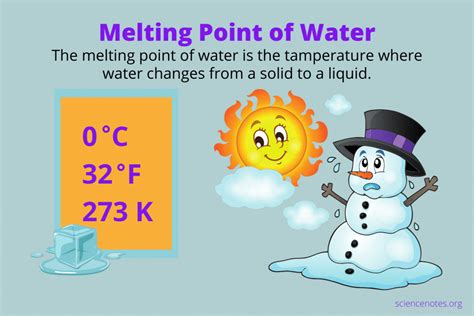
Table of Contents
Melting Point Temperature in Celsius for Water: A Deep Dive
Water, a seemingly simple molecule (H₂O), exhibits remarkably complex properties, many of which are crucial for life on Earth. One of the most fundamental properties is its melting point, the temperature at which it transitions from a solid (ice) to a liquid. This article delves deep into the melting point temperature of water in Celsius, exploring the factors that influence it, its significance in various fields, and the nuances behind its seemingly simple value.
Understanding the Melting Point
The melting point of a substance is the temperature at which its solid and liquid phases coexist in equilibrium at a given pressure. At this point, the energy supplied to the substance is used to overcome the intermolecular forces holding the molecules in a rigid, crystalline structure (ice), allowing them to move more freely as a liquid. For water, under standard atmospheric pressure (1 atmosphere or 101.325 kPa), this transition occurs precisely at 0° Celsius (or 32° Fahrenheit).
Factors Affecting the Melting Point of Water
While 0°C is the standard melting point, several factors can subtly influence it:
-
Pressure: This is perhaps the most significant factor influencing the melting point of water. Unlike most substances, the melting point of water decreases with increasing pressure. This unusual behavior is a consequence of the unique structure of ice, where the hydrogen bonds create a less dense structure than liquid water. Applying pressure forces the molecules closer together, favoring the denser liquid phase and thus lowering the melting point. This effect is relatively small but measurable.
-
Impurities: The presence of dissolved substances (solutes) in water, such as salts or sugars, can lower its freezing point (and consequently its melting point), a phenomenon known as freezing point depression. This occurs because the solute molecules interfere with the formation of the ice crystal lattice, requiring a lower temperature to initiate the phase transition. The extent of depression depends on the concentration of the solute. This is why saltwater has a lower freezing point than pure water and why we use salt to de-ice roads in winter.
-
Isotopic Composition: Water molecules can be composed of different isotopes of hydrogen (protium, deuterium, tritium) and oxygen. The presence of heavier isotopes, such as deuterium, increases the intermolecular forces, leading to a slightly higher melting point. Heavy water (D₂O) has a melting point of approximately 3.8°C.
-
Electric and Magnetic Fields: While the effect is minor, extremely strong electric and magnetic fields can subtly influence the molecular interactions in water, leading to slight variations in the melting point. This is an area of ongoing research and has limited practical implications.
The Significance of Water's Melting Point
The precise melting point of water at 0°C has far-reaching consequences across numerous scientific disciplines and everyday life:
-
Climate Regulation: The melting and freezing of water plays a vital role in regulating Earth's climate. The high heat capacity of water moderates temperature fluctuations, while the melting and freezing of ice absorb and release significant amounts of energy (latent heat), influencing weather patterns and climate stability.
-
Biological Processes: The fact that water melts at 0°C is crucial for life as we know it. Many biological processes occur within a narrow temperature range around 0°C, and the relatively high melting point ensures that liquid water is abundant in many environments, supporting life. The unique properties of water, such as its high specific heat capacity and surface tension, all tie back to its molecular structure and its behavior around the melting point.
-
Environmental Science: The melting and freezing of water are critical processes in various environmental contexts, including hydrology, glaciology, and oceanography. The melting of glaciers and polar ice caps, driven by rising temperatures, has profound implications for sea level rise and global climate change. Understanding the nuances of water's melting point is crucial for predicting and mitigating the effects of these changes.
-
Chemistry and Material Science: Water’s melting point serves as a fundamental reference point in many chemical and materials science experiments and processes. Calibration of thermometers, development of cryogenic techniques, and study of phase transitions all rely on the precise knowledge of the melting point.
-
Engineering and Technology: Understanding how impurities and pressure affect the melting point of water is essential in engineering applications, such as designing cooling systems, preventing ice formation in pipes, and developing materials that perform effectively under varying temperature conditions.
Measuring the Melting Point of Water
Precisely determining the melting point of water requires careful experimental setup and meticulous measurements. The procedure generally involves:
-
Sample Preparation: Using highly purified water is crucial to minimize the effects of impurities. The water sample should be free from dissolved gases and other contaminants.
-
Temperature Control: A highly accurate thermometer, calibrated against a known standard, is essential for precise temperature measurements. A controlled environment is necessary to maintain stable temperatures.
-
Observation of Phase Change: The phase transition from solid to liquid is typically observed visually, noting the temperature at which the ice begins to melt and the temperature at which all the ice has melted. This range indicates the melting point, and the average temperature can be calculated.
-
Pressure Control: For highly precise measurements, the ambient pressure must be carefully monitored and controlled, as pressure variations can influence the melting point.
-
Data Analysis: Multiple measurements are conducted to ensure reproducibility and calculate an average melting point. Statistical analysis helps determine the uncertainty associated with the measurement.
Beyond 0°C: Variations and Anomalies
While 0°C is the standard melting point of water, it’s crucial to remember that this is a simplification. Under non-standard conditions, the melting point can deviate. The factors already discussed—pressure, impurities, isotopic composition—all contribute to variations. For example:
-
Supercooling: Water can sometimes remain in a liquid state below 0°C, a phenomenon known as supercooling. This occurs when the water lacks nucleation sites, such as dust particles or imperfections in the container, necessary for the formation of ice crystals. Supercooled water is metastable and can quickly freeze upon the introduction of a nucleation site or even a slight disturbance.
-
Ice polymorphism: Water can exist in various crystalline forms (polymorphs) of ice, each with slightly different properties and melting points. Ice Ih (the common hexagonal ice) melts at 0°C under standard conditions, but other polymorphs, such as Ice II, Ice III, and Ice VII, exist under high pressure conditions and have different melting points.
-
Water confined in nano-spaces: The melting point of water can also change drastically when confined within nano-scale spaces, such as within carbon nanotubes or porous materials. In these conditions, the interfacial interactions between the water molecules and the confining material influence the melting behavior. These changes in melting point can be significant and are areas of ongoing scientific research.
Conclusion: The Importance of Precision and Context
The melting point of water at 0°C is a fundamental constant in science and everyday life. However, the seemingly simple value hides a wealth of complexity, influenced by various factors and exhibiting unexpected behavior under specific conditions. Understanding these intricacies is critical for applications ranging from climate modeling to materials science. Precision in measurement and consideration of context are essential when dealing with the melting point of water. Continued research into the subtle variations and anomalies will further enhance our understanding of this essential property of a molecule that underpins life on Earth.
Latest Posts
Latest Posts
-
Least Common Multiple Of 6 And 14
Mar 04, 2025
-
Common Factors Of 27 And 18
Mar 04, 2025
-
What Is The Lcm Of 12 And 5
Mar 04, 2025
-
What Is The Lcm Of 7 And 6
Mar 04, 2025
-
How Many Legs Does An Ant Has
Mar 04, 2025
Related Post
Thank you for visiting our website which covers about Melting Point Temperature In Celsius For Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
