Matter Is Anything That Has Mass And
Juapaving
Mar 24, 2025 · 6 min read
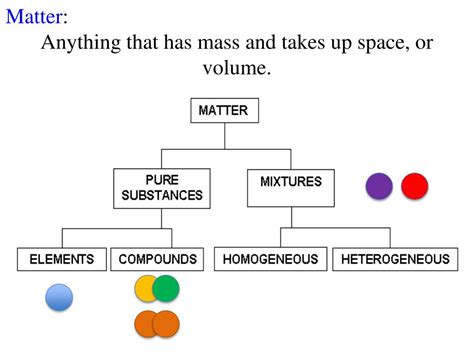
Table of Contents
Matter: Anything That Has Mass and Occupies Space
The universe, in all its vastness and complexity, is fundamentally composed of matter. But what exactly is matter? At its most basic definition, matter is anything that has mass and occupies space. This seemingly simple statement underpins our understanding of the physical world, from the smallest subatomic particles to the largest galaxies. This article will delve deep into the concept of matter, exploring its properties, classifications, states, and the fundamental forces that govern its behavior.
Understanding Mass and Volume
Before we dive deeper into the intricacies of matter, let's clarify the two key characteristics that define it: mass and volume.
Mass: A Measure of Inertia
Mass is a measure of an object's inertia – its resistance to changes in motion. A more massive object requires a greater force to accelerate it than a less massive object. It's crucial to differentiate mass from weight. While weight is the force of gravity acting on an object's mass, mass remains constant regardless of gravitational pull. An astronaut on the moon weighs less than on Earth due to the weaker lunar gravity, but their mass remains the same.
Volume: The Space Occupied
Volume refers to the amount of three-dimensional space occupied by an object or substance. It's often expressed in cubic units, such as cubic meters (m³) or cubic centimeters (cm³). Understanding volume is crucial in determining the density of a substance, which is the mass per unit volume.
The States of Matter
Matter exists in various states, each characterized by different properties and arrangements of its constituent particles. The most common states are:
Solid
Solids have a definite shape and volume. Their particles are tightly packed together in a fixed arrangement, resulting in strong intermolecular forces. This explains their rigidity and resistance to compression. Examples include ice, rocks, and wood.
Liquid
Liquids have a definite volume but take the shape of their container. Their particles are closer together than in gases but are not rigidly fixed in place, allowing them to flow and adapt to the shape of their surroundings. Examples include water, oil, and mercury.
Gas
Gases have neither a definite shape nor volume. Their particles are widely dispersed and move freely, resulting in weak intermolecular forces. Gases readily expand to fill their containers and are easily compressible. Examples include air, oxygen, and carbon dioxide.
Plasma
Plasma, often considered the fourth state of matter, is a highly energized state where electrons are stripped from atoms, creating a mixture of ions and free electrons. It's characterized by its high electrical conductivity and responsiveness to magnetic fields. Plasma is found in stars, lightning, and fluorescent lights.
Bose-Einstein Condensate (BEC)
Bose-Einstein Condensates are a fascinating state of matter that occurs at extremely low temperatures, near absolute zero. In this state, a large fraction of the atoms occupy the lowest quantum state, behaving as a single quantum entity. BECs exhibit unique properties and are of significant interest in quantum physics research.
Other Exotic States
Beyond these common states, there are other exotic states of matter, including:
- Superfluids: Liquids that flow without any viscosity.
- Superconductors: Materials that conduct electricity with zero resistance.
- Quark-gluon plasma: A state of matter that existed shortly after the Big Bang, composed of free quarks and gluons.
These exotic states often exhibit unusual properties and are the subject of ongoing research.
Classification of Matter
Matter can also be classified based on its composition:
Pure Substances
Pure substances are composed of only one type of atom or molecule. They have a fixed composition and consistent properties. Pure substances can be further divided into:
-
Elements: Elements are the fundamental building blocks of matter, composed of only one type of atom. Examples include oxygen (O), hydrogen (H), and iron (Fe). The periodic table organizes and classifies all known elements.
-
Compounds: Compounds are formed when two or more different elements chemically combine in a fixed ratio. Water (H₂O), for instance, is a compound formed from two hydrogen atoms and one oxygen atom. Compounds have properties that differ significantly from their constituent elements.
Mixtures
Mixtures are combinations of two or more substances that are not chemically bonded. They retain the individual properties of their components and can be separated by physical means. Mixtures are classified into:
-
Homogeneous Mixtures: In homogeneous mixtures, the components are uniformly distributed throughout the mixture, resulting in a uniform appearance. Examples include saltwater and air.
-
Heterogeneous Mixtures: In heterogeneous mixtures, the components are not uniformly distributed, resulting in a visibly non-uniform appearance. Examples include sand and water, and a salad.
The Fundamental Forces of Matter
The behavior of matter is governed by four fundamental forces:
Strong Nuclear Force
The strong nuclear force is the strongest of the four forces. It acts within the nucleus of an atom, binding protons and neutrons together. Without this force, atomic nuclei would disintegrate.
Electromagnetic Force
The electromagnetic force governs the interactions between electrically charged particles. It's responsible for the attraction between electrons and protons, holding atoms together, and for many other phenomena such as light and magnetism.
Weak Nuclear Force
The weak nuclear force is responsible for radioactive decay, a process where unstable atomic nuclei transform into more stable ones. It plays a crucial role in nuclear reactions within stars.
Gravitational Force
Gravity is the weakest of the four fundamental forces but acts over vast distances. It's responsible for the attraction between objects with mass, holding planets in orbit around stars and stars together in galaxies.
The Atomic Structure and Subatomic Particles
To fully understand matter, it's essential to explore its atomic structure. Atoms are the fundamental units of elements, consisting of a nucleus containing protons and neutrons, surrounded by orbiting electrons.
Protons
Protons are positively charged subatomic particles found in the nucleus. The number of protons in an atom's nucleus determines its atomic number and identifies the element.
Neutrons
Neutrons are neutral particles (no charge) also found in the nucleus. They contribute to the atom's mass but not its charge.
Electrons
Electrons are negatively charged particles that orbit the nucleus. Their number typically equals the number of protons in a neutral atom. Electrons are involved in chemical bonding and determine an atom's chemical properties.
Subatomic Particles and Quantum Mechanics
The realm of subatomic particles extends beyond protons, neutrons, and electrons. Quantum mechanics describes the behavior of these particles, revealing a world governed by probabilities and uncertainties. Quarks, leptons, and bosons are examples of fundamental particles that make up matter and mediate fundamental forces.
The Importance of Understanding Matter
Understanding matter is fundamental to many scientific disciplines, including chemistry, physics, and materials science. It's crucial for:
-
Developing new materials: Understanding the properties of matter allows scientists to design and create new materials with specific properties, such as strength, conductivity, or reactivity.
-
Advancing medicine: Understanding the interactions between molecules is crucial for developing new drugs and treatments.
-
Solving environmental problems: Understanding the behavior of matter in the environment is essential for addressing issues such as pollution and climate change.
-
Exploring the universe: Understanding the composition and behavior of matter is crucial for understanding the formation and evolution of stars, galaxies, and the universe itself.
Conclusion
Matter, in its various forms and states, is the fundamental building block of the universe. Its properties, classifications, and the forces that govern its behavior are central to our understanding of the physical world. From the smallest subatomic particles to the largest cosmic structures, matter's intricacies continue to fascinate and challenge scientists, pushing the boundaries of our knowledge and leading to groundbreaking discoveries that shape our world. Further exploration into the mysteries of matter promises to unlock even deeper insights into the fundamental nature of reality.
Latest Posts
Latest Posts
-
900 1800 2700 3600 4500 5400 6300 7200 8100 9000
Mar 26, 2025
-
What Are All Of The Factors Of 84
Mar 26, 2025
-
Is Carbon Metal Or Non Metal
Mar 26, 2025
-
What Is The Lcm For 6 And 12
Mar 26, 2025
-
What Is A Multiple Of 24
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Matter Is Anything That Has Mass And . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
