Magnesium And Hydrochloric Acid Balanced Equation
Juapaving
Mar 09, 2025 · 5 min read
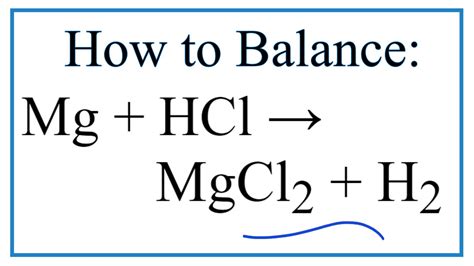
Table of Contents
Magnesium and Hydrochloric Acid: A Balanced Equation and Beyond
Magnesium reacting with hydrochloric acid is a classic example of a single displacement reaction, a cornerstone of introductory chemistry. Understanding this reaction, from its balanced equation to its applications and safety considerations, is crucial for anyone studying chemistry or related fields. This in-depth article explores the reaction in detail, covering its stoichiometry, the observations you'd make in a lab setting, its applications, safety precautions, and extensions to related concepts.
The Balanced Chemical Equation
The reaction between magnesium (Mg) and hydrochloric acid (HCl) produces magnesium chloride (MgCl₂) and hydrogen gas (H₂). The balanced chemical equation representing this reaction is:
Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g)
This equation signifies that one mole of solid magnesium reacts with two moles of aqueous hydrochloric acid to produce one mole of aqueous magnesium chloride and one mole of hydrogen gas. The (s), (aq), and (g) indicate the physical states: solid, aqueous (dissolved in water), and gas, respectively. This balancing is critical for stoichiometric calculations, ensuring the law of conservation of mass is upheld – the number of atoms of each element remains the same on both sides of the equation.
Understanding the Reaction Mechanism
The reaction proceeds through a single displacement mechanism. The more reactive magnesium metal displaces the hydrogen from the hydrochloric acid. The hydrogen ions (H⁺) in the HCl solution gain electrons from the magnesium, reducing them to hydrogen gas (H₂). Simultaneously, the magnesium atoms lose electrons, oxidizing them to magnesium ions (Mg²⁺), which then combine with the chloride ions (Cl⁻) to form magnesium chloride.
Observations in a Laboratory Setting
Performing this reaction in a laboratory allows for firsthand observation of the chemical changes. Here's what you would typically see:
- Brisk Effervescence: The most noticeable observation is the vigorous bubbling, or effervescence, indicating the evolution of hydrogen gas. The rate of bubbling will depend on the concentration of the acid and the surface area of the magnesium. A finely powdered magnesium will react faster than a large magnesium ribbon.
- Dissolution of Magnesium: As the reaction proceeds, the magnesium metal will gradually dissolve into the solution. The solid magnesium will diminish in size or disappear entirely depending on the amount of reactants used.
- Temperature Increase: The reaction is exothermic, meaning it releases heat. You'll notice a temperature increase in the solution. This can be easily measured using a thermometer.
- Change in Solution Appearance: The solution will initially be clear (if using dilute HCl). As the reaction proceeds, the solution might become slightly cloudy depending on the purity of the magnesium and the presence of any impurities. In many cases, it remains clear.
Stoichiometric Calculations and Applications
The balanced equation is essential for performing various stoichiometric calculations. For instance, given the mass of magnesium, you can calculate the volume of hydrogen gas produced (at standard temperature and pressure, or STP) using the molar mass of magnesium and the ideal gas law (PV=nRT). Similarly, you can determine the mass of magnesium chloride formed. These calculations are fundamental in quantitative analysis and chemical engineering.
The reaction itself has several applications:
- Hydrogen Gas Production: This reaction is a simple and convenient method for producing small quantities of hydrogen gas in a laboratory setting. Hydrogen is a crucial element in many industrial processes and has applications in fuel cells and other energy technologies. While not a large-scale production method due to cost and safety concerns, its use in laboratory demonstrations and small-scale experiments is invaluable.
- Cleaning Metal Surfaces: The reaction can be used to clean metal surfaces by removing oxides or other impurities. This is not a common industrial application but could be useful in certain specialized scenarios.
- Educational Purposes: The magnesium-hydrochloric acid reaction is widely used in educational settings to demonstrate the principles of chemical reactions, stoichiometry, and gas laws. Its relatively straightforward nature and observable changes make it ideal for teaching purposes.
Safety Precautions
It's crucial to emphasize the importance of safety precautions when working with hydrochloric acid and magnesium:
- Eye Protection: Always wear safety goggles to protect your eyes from splashes of the corrosive hydrochloric acid.
- Gloves: Use chemical-resistant gloves to prevent skin contact with the acid. Hydrochloric acid can cause burns and irritation.
- Ventilation: Perform the reaction in a well-ventilated area or under a fume hood to prevent inhalation of hydrogen gas, which is flammable.
- Acid Handling: Handle hydrochloric acid with care, avoiding spills and contact with clothing. Neutralize any spills immediately using a suitable base like sodium bicarbonate.
- Fire Safety: Keep away from open flames as hydrogen gas is highly flammable and can form explosive mixtures with air.
- Waste Disposal: Dispose of the reaction mixture properly according to your institution’s guidelines. Never pour acid down the drain without proper neutralization.
Extensions and Related Concepts
Understanding the magnesium and hydrochloric acid reaction opens doors to exploring related concepts:
- Reaction Rates: The reaction rate can be investigated by varying the concentration of the acid, the surface area of magnesium, or the temperature. This allows for a practical exploration of factors influencing reaction kinetics.
- Thermochemistry: Measuring the temperature change during the reaction allows for the calculation of the enthalpy change (ΔH), providing insights into the thermodynamics of the reaction. Is it exothermic or endothermic? What is its energy change?
- Electrochemistry: The reaction can be adapted to a galvanic cell, demonstrating the principles of electrochemistry and generating an electric current.
- Redox Reactions: This reaction is a classic example of a redox (reduction-oxidation) reaction, where magnesium is oxidized and hydrogen ions are reduced. This illustrates the fundamental concept of electron transfer in chemical reactions.
- Titration: The concentration of the hydrochloric acid can be determined using a titration method with a standard base. The reaction provides the basis for quantitative analysis through this method.
Conclusion
The reaction between magnesium and hydrochloric acid, while seemingly simple, provides a rich learning experience encompassing various aspects of chemistry. From balancing the chemical equation and performing stoichiometric calculations to understanding reaction mechanisms and safety procedures, this reaction serves as a fundamental building block for more advanced concepts. Its applications, while limited in large-scale industry, are significant in laboratory settings, educational demonstrations, and for illustrating core chemical principles. Remember always to prioritize safety when conducting experiments involving chemicals.
Latest Posts
Latest Posts
-
The Correct Name For The Compound N2o3 Is
Mar 09, 2025
-
Which Of The Following Is A Function Of The Nucleus
Mar 09, 2025
-
What Is The Roman Numeral For 59
Mar 09, 2025
-
What Are Three Types Of Ecological Pyramids
Mar 09, 2025
-
What Is The Magnitude Of Displacement
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about Magnesium And Hydrochloric Acid Balanced Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
