List Three Physical Properties Of Ionic Compounds
Juapaving
Mar 28, 2025 · 6 min read
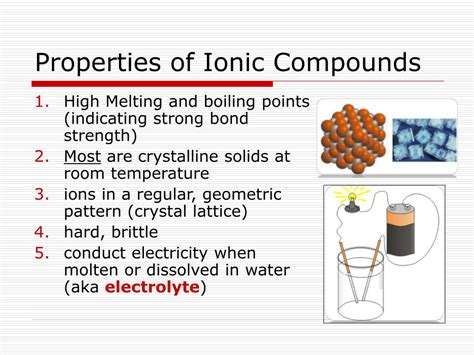
Table of Contents
List Three Physical Properties of Ionic Compounds: A Deep Dive
Ionic compounds, formed through the electrostatic attraction between oppositely charged ions, exhibit a unique set of physical properties that distinguish them from other types of compounds like covalent compounds or metallic compounds. Understanding these properties is crucial in various fields, from materials science and chemistry to geology and environmental science. This article delves into three key physical properties of ionic compounds: high melting and boiling points, brittleness, and electrical conductivity. We'll explore the underlying reasons for these characteristics and examine their implications.
1. High Melting and Boiling Points: The Strong Grip of Electrostatic Forces
One of the most defining characteristics of ionic compounds is their exceptionally high melting and boiling points. This property directly stems from the strong electrostatic forces of attraction between the positively charged cations and the negatively charged anions within the ionic lattice. These forces, often referred to as ionic bonds, are significantly stronger than the intermolecular forces found in covalent or metallic compounds.
The Ionic Lattice: A Crystal Structure of Strength
Ionic compounds don't exist as individual molecules; instead, they form a three-dimensional crystal lattice structure. This lattice is a highly ordered arrangement of ions, with cations and anions alternating to maximize electrostatic attraction and minimize repulsion. The strength of this lattice is directly proportional to the charge of the ions and inversely proportional to the distance between them. The higher the charges and the smaller the ions, the stronger the electrostatic attraction, resulting in a higher melting and boiling point.
Overcoming the Strong Bonds: The Energy Barrier
To melt or boil an ionic compound, you need to supply sufficient energy to overcome these strong electrostatic forces holding the ions together in the lattice. This requires a significant amount of heat energy, leading to the high melting and boiling points observed. For example, sodium chloride (NaCl), common table salt, has a melting point of 801°C and a boiling point of 1413°C, showcasing the strength of the ionic bonds within its crystal lattice.
Factors Influencing Melting and Boiling Points: Charge and Size
Several factors influence the magnitude of the melting and boiling points in ionic compounds:
-
Charge of the ions: Higher charges on the ions lead to stronger electrostatic attraction and thus higher melting and boiling points. For instance, magnesium oxide (MgO) with Mg²⁺ and O²⁻ ions has a much higher melting point than sodium chloride (NaCl) with Na⁺ and Cl⁻ ions.
-
Size of the ions: Smaller ions lead to a shorter distance between the nuclei and hence stronger electrostatic attraction. This results in higher melting and boiling points. For example, lithium fluoride (LiF) with smaller ions has a higher melting point than sodium fluoride (NaF).
-
Lattice structure: The specific arrangement of ions in the crystal lattice can also influence the melting and boiling points. Different lattice structures exhibit varying degrees of stability.
Applications and Implications: From Metallurgy to Ceramics
The high melting and boiling points of ionic compounds have numerous practical applications:
-
High-temperature applications: Many ionic compounds are used in high-temperature applications such as furnace linings, refractory materials, and heat shields due to their ability to withstand extreme temperatures without melting or decomposing.
-
Ceramics: Many ceramics are based on ionic compounds, leveraging their high melting points and hardness to create durable and heat-resistant materials used in various applications, from construction to electronics.
-
Metallurgy: Ionic compounds are essential in metallurgical processes, such as the extraction and refining of metals. Their high melting points allow them to be used as fluxes or solvents in high-temperature reactions.
2. Brittleness: A Consequence of Crystal Structure
Another prominent characteristic of ionic compounds is their brittleness. Unlike metals, which are ductile and malleable, ionic compounds are easily shattered when subjected to stress. This brittleness is directly linked to their crystal structure and the nature of ionic bonds.
The Impact of Stress: Disrupting the Lattice
When an external force is applied to an ionic crystal, the layers of ions within the lattice can shift. This shift can bring similarly charged ions into close proximity, leading to strong repulsive forces. These repulsive forces overcome the attractive forces holding the crystal together, causing the crystal to fracture or shatter along cleavage planes.
Cleavage Planes: Weak Points in the Crystal
The cleavage planes are areas where the repulsive forces are maximized, essentially representing the weakest points within the crystal structure. When subjected to stress, these planes tend to break, leading to the characteristic brittleness. This contrasts sharply with metals, which can deform under stress due to the delocalized nature of their electrons.
Implications and Applications: Careful Handling and Specialized Uses
The brittleness of ionic compounds needs to be considered in many applications:
-
Material Handling: Ionic compounds require careful handling to avoid breakage and fragmentation.
-
Specialized Applications: Although brittleness is a limitation in many cases, it also finds niche applications. The ability to fracture along clean planes can be exploited in some processes.
-
Ceramic Engineering: Ceramic engineers need to account for brittleness in the design and manufacturing processes. Techniques to enhance toughness are often employed.
3. Electrical Conductivity: The Role of Ions and the State of Matter
The electrical conductivity of ionic compounds depends heavily on their state of matter. In the solid state, ionic compounds are generally poor conductors of electricity. However, they become good conductors when molten (liquid) or dissolved in water (aqueous solution).
Solid State: Immobile Ions
In the solid state, the ions are held tightly in the crystal lattice and are relatively immobile. Therefore, they cannot move freely to carry an electric current. This immobility of ions results in low electrical conductivity in the solid state.
Molten State and Aqueous Solutions: Mobile Ions
However, when ionic compounds are heated to their melting point, the crystal lattice breaks down, and the ions become free to move. These mobile ions can then carry an electric charge, leading to significantly increased electrical conductivity. Similarly, when ionic compounds are dissolved in water, the ions dissociate and become mobile, leading to enhanced electrical conductivity.
Electrolytes: The Foundation of Electrochemical Processes
Ionic compounds that conduct electricity when molten or dissolved in water are known as electrolytes. Electrolytes play a crucial role in many electrochemical processes, including:
-
Batteries: Electrolytes are essential components of batteries, facilitating the movement of ions between electrodes and enabling the generation of electrical energy.
-
Electroplating: Electrolytes are used in electroplating processes to deposit a thin layer of metal onto a conductive surface.
-
Corrosion: Electrolytes can play a role in corrosion processes, facilitating the electrochemical reactions that lead to the degradation of metals.
Conclusion: Understanding the Properties of Ionic Compounds
The three physical properties discussed—high melting and boiling points, brittleness, and electrical conductivity—are intrinsically linked to the ionic bonding and crystal structure of these compounds. Understanding these properties is paramount in various scientific and engineering disciplines. Their unique characteristics lead to a wide range of applications, from high-temperature materials to electrochemical processes, emphasizing the importance of ionic compounds in our daily lives and technological advancements. Further exploration of these properties and their influence on material behavior continues to drive innovation across multiple fields. The interplay between the charge, size, and arrangement of ions within the crystal lattice provides a rich area for continued research and technological development.
Latest Posts
Latest Posts
-
What Are 5 Examples Of Chemical Weathering
Mar 31, 2025
-
5 Letter Words Starting With H A I
Mar 31, 2025
-
How Many Mm Are In One Meter
Mar 31, 2025
-
Does A Flatworm Have A Coelom
Mar 31, 2025
-
An Automobile Engine Converts Energy Into Energy
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about List Three Physical Properties Of Ionic Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
