List The Three Principles Of The Kinetic Molecular Theory
Juapaving
Mar 24, 2025 · 7 min read
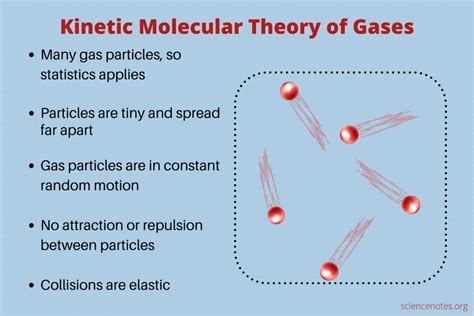
Table of Contents
The Three Pillars of Kinetic Molecular Theory: A Deep Dive into Gas Behavior
The Kinetic Molecular Theory (KMT) is a cornerstone of chemistry, providing a powerful framework for understanding the behavior of gases. While often simplified to include five postulates, the core essence of KMT rests on three fundamental principles: constant, random motion of particles; negligible interparticle forces; and negligible particle volume. These principles, when considered together, elegantly explain macroscopic gas properties like pressure, volume, and temperature. Let's delve into each of these pillars in detail, exploring their implications and limitations.
1. Constant, Random Motion of Particles: The Driving Force Behind Gas Behavior
This principle forms the very foundation of KMT. It posits that gas particles – be they atoms or molecules – are in constant, ceaseless, and random motion. This isn't just a gentle jiggle; it's a dynamic dance of high-speed collisions. These particles are not static; they're constantly traversing space, impacting each other and the walls of their container.
Understanding the Implications:
- Pressure: The incessant bombardment of gas particles against the container walls is what generates pressure. The more frequent and forceful these collisions, the higher the pressure. This directly links microscopic particle motion to a macroscopic observable.
- Temperature: Temperature is directly proportional to the average kinetic energy of the gas particles. Higher temperatures mean faster particle speeds and more energetic collisions. This explains why gases expand when heated; increased kinetic energy leads to more forceful collisions and expansion to occupy more space.
- Diffusion and Effusion: The constant, random motion explains the phenomena of diffusion (the spreading of gases) and effusion (the escape of gases through a small hole). Particles constantly move and collide, leading to a net movement from regions of high concentration to regions of low concentration (diffusion) and escape from the container (effusion).
Beyond the Ideal:
While the assumption of constant, random motion is central to the ideal gas law, real gases deviate from this ideal behavior at high pressures and low temperatures. At high pressures, the interparticle forces become significant, affecting the freedom of motion. At low temperatures, the kinetic energy is reduced, causing particles to move slower and interact more strongly. These deviations lead to the need for more complex equations of state like the van der Waals equation to accurately model real gas behavior.
2. Negligible Interparticle Forces: The Ideal Gas Assumption
The second fundamental principle asserts that the forces of attraction and repulsion between gas particles are negligible. This means that the particles are essentially independent of each other; they don't interact significantly except during brief collisions.
The Ideal Gas Model and its Limitations:
This assumption is crucial for the ideal gas law (PV=nRT). The ideal gas law works remarkably well for many gases under standard conditions because the interparticle forces are relatively weak. However, this approximation breaks down when:
- Pressure is high: At high pressures, the gas particles are forced closer together, leading to an increase in the strength of attractive forces. This results in a decrease in the observed volume compared to the ideal gas prediction, because the attractive forces partially overcome the kinetic energy.
- Temperature is low: At low temperatures, the kinetic energy of the particles is reduced, making the weak interparticle attractive forces more significant. These forces can lead to condensation (the transition from gas to liquid) as the particles overcome their kinetic energy and stick together more strongly.
- Polar molecules are involved: Gases composed of polar molecules (molecules with a permanent dipole moment) exhibit stronger intermolecular forces (dipole-dipole interactions, hydrogen bonding) than nonpolar gases. These forces cause significant deviations from the ideal gas behavior, particularly at lower temperatures and higher pressures.
Understanding Real Gas Behavior:
To account for the non-negligible intermolecular forces, modifications to the ideal gas law are necessary. The van der Waals equation is a notable example. It incorporates correction terms to account for the volume occupied by the particles themselves and the attractive forces between them, offering a more accurate description of real gas behavior.
3. Negligible Particle Volume: Points vs. Spheres
The third crucial postulate of KMT states that the volume occupied by the gas particles themselves is negligible compared to the total volume of the container. This means that we can essentially treat gas particles as point masses, devoid of any significant volume.
Implications and Limitations:
This assumption simplifies calculations considerably. In the ideal gas model, the entire volume of the container is assumed to be available for the gas particles to move in. However, real gas particles do have a finite volume.
- High-Pressure Scenarios: At high pressures, the volume occupied by the gas particles becomes a significant fraction of the total volume. This leads to a reduction in the available space for the particles to move, causing deviations from the ideal gas law. The particles are essentially bumping into each other more than they would in a true ideal case.
- Real Gases vs. Ideal Gases: The difference between real and ideal gas behavior becomes more pronounced at higher pressures because the volume occupied by the particles can no longer be ignored. The space that the particles actually occupy must be accounted for to get a more accurate understanding of the situation.
Combining the Principles: A Holistic View
These three principles – constant random motion, negligible intermolecular forces, and negligible particle volume – paint a picture of an idealized gas. While real gases deviate from this ideal behavior, particularly under extreme conditions, KMT provides an invaluable foundation for understanding gas behavior. The model allows us to explain and predict many macroscopic gas properties based on the microscopic motion of individual particles. Understanding the limitations of these assumptions helps us bridge the gap between the idealized world of KMT and the complexities of real gas behavior.
Beyond the Three Pillars: Expanding the KMT Understanding
While the three principles discussed above form the core of KMT, a complete understanding usually includes two additional postulates often presented together with the three pillars:
-
Elastic Collisions: Collisions between gas particles and between particles and the container walls are assumed to be perfectly elastic. This means that there is no loss of kinetic energy during collisions; the total kinetic energy is conserved. This is, of course, an idealization. In reality, some energy is lost as heat during collisions.
-
No Intermolecular Forces (Revisited): This is a crucial postulate already mentioned. The attractive or repulsive forces between gas molecules are negligible. This assumption is necessary to simplify the calculations and is essential for the validity of the ideal gas law. As we discussed above, this assumption breaks down under certain conditions.
By considering these five postulates as a whole, we gain a more comprehensive model of gas behavior. This complete picture allows for a deeper understanding of phenomena such as diffusion, effusion, and the relationship between pressure, volume, temperature, and the amount of gas. It also lays the foundation for understanding deviations from ideal behavior and the development of more sophisticated models to account for those deviations.
Conclusion: KMT – A Powerful, Approximative Model
The Kinetic Molecular Theory, with its three fundamental principles (and the two additional postulates often included), is a powerful, albeit approximate, model for understanding gas behavior. The beauty of KMT lies in its ability to connect the microscopic world of individual particles to the macroscopic world of observable properties. While it simplifies reality by neglecting interparticle forces and particle volume, it provides an excellent starting point for understanding gas laws and lays the foundation for more sophisticated models that take into account the complexities of real gases. By understanding the limitations of KMT, we can appreciate its strength as a foundational concept in chemistry and physics. It allows for a rich understanding of gas behavior and a robust framework for predicting and interpreting experimental observations.
Latest Posts
Latest Posts
-
Whats The Smallest Organ In The Human Body
Mar 26, 2025
-
Aqua Regia Is A Mixture Of
Mar 26, 2025
-
What Is The Natural Boundary Between France And Italy
Mar 26, 2025
-
Which Of The Following Is Not A Pyrimidine
Mar 26, 2025
-
What Is The Boiling Point Of Blood
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about List The Three Principles Of The Kinetic Molecular Theory . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
