List The Three Parts Of A Nucleotide
Juapaving
Mar 13, 2025 · 7 min read
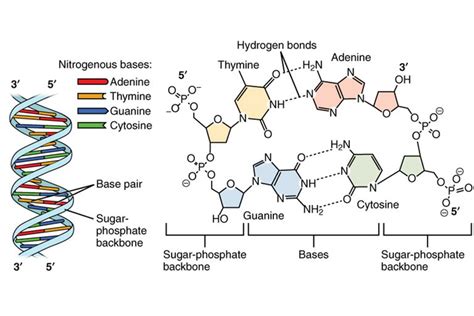
Table of Contents
Decoding the Building Blocks of Life: A Deep Dive into Nucleotides and Their Three Essential Parts
Nucleotides: the very name conjures images of complex biological machinery, the intricate workings of DNA and RNA. But what are these fundamental units, and what makes them so crucial for life as we know it? Understanding nucleotides requires delving into their core components – the three parts that combine to form these essential building blocks of nucleic acids. This article provides a comprehensive exploration of these three parts, delving into their chemical properties, their roles in nucleic acid structure, and their broader significance in cellular processes.
The Trinity of Nucleotides: Sugar, Base, and Phosphate
Every nucleotide, irrespective of its role in DNA, RNA, or other crucial cellular processes, consists of three fundamental components:
- A Pentose Sugar: This five-carbon sugar forms the backbone of the nucleotide structure.
- A Nitrogenous Base: This component is responsible for the genetic information encoded within nucleic acids.
- A Phosphate Group: This negatively charged group plays a crucial role in the nucleotide's chemical properties and its ability to link with other nucleotides.
Let's delve into each component individually, exploring their specific features and functions.
1. The Pentose Sugar: The Sweet Backbone of Nucleotides
The sugar component in a nucleotide is a pentose, meaning it's a five-carbon sugar. However, the specific pentose sugar differs between DNA and RNA. This seemingly small difference has profound implications for the structure and function of these two nucleic acids.
Deoxyribose in DNA:
In DNA (deoxyribonucleic acid), the pentose sugar is deoxyribose. The "deoxy" prefix signifies that deoxyribose lacks an oxygen atom on the 2' carbon compared to ribose, the sugar found in RNA. This seemingly minor chemical difference drastically affects the molecule's stability and overall structure. The absence of the hydroxyl group (–OH) at the 2' carbon in deoxyribose makes DNA a more stable molecule, better suited for long-term storage of genetic information. The increased stability of DNA is critical for preserving the integrity of the genome across generations.
Ribose in RNA:
In RNA (ribonucleic acid), the pentose sugar is ribose. Ribose contains a hydroxyl group (–OH) at the 2' carbon, making it more reactive than deoxyribose. This increased reactivity contributes to RNA's greater versatility in various cellular roles. RNA is involved in a wide array of functions beyond simply storing genetic information, including protein synthesis, gene regulation, and even catalyzing chemical reactions (ribozymes). The presence of the 2'-hydroxyl group influences RNA's secondary structure, making it more prone to folding into complex three-dimensional shapes.
Key Differences and their Significance: The presence or absence of the hydroxyl group at the 2' carbon is a crucial differentiator between DNA and RNA, contributing to their unique properties and biological roles. The increased stability of DNA's deoxyribose makes it ideally suited for long-term genetic information storage, while the greater reactivity of RNA's ribose allows for its diverse functional roles within the cell.
2. The Nitrogenous Base: The Alphabet of Genetics
The nitrogenous base is the information-carrying component of a nucleotide. These bases are organic molecules containing nitrogen and are categorized into two main groups: purines and pyrimidines.
Purines:
Purines are larger, double-ringed structures. There are two purine bases found in both DNA and RNA:
- Adenine (A): A crucial base paired with thymine (in DNA) or uracil (in RNA).
- Guanine (G): Paired with cytosine in both DNA and RNA.
Pyrimidines:
Pyrimidines are smaller, single-ringed structures. The pyrimidine bases differ slightly between DNA and RNA:
- Cytosine (C): Found in both DNA and RNA, always pairing with guanine.
- Thymine (T): Found only in DNA, pairing with adenine.
- Uracil (U): Found only in RNA, pairing with adenine.
Base Pairing: The specific pairing of bases (A with T/U, and G with C) is fundamental to the double helix structure of DNA and the various secondary structures adopted by RNA. These base pairs are held together by hydrogen bonds, which are relatively weak but collectively provide stability to the nucleic acid structures.
Genetic Code: The sequence of nitrogenous bases along a nucleic acid strand constitutes the genetic code. This code dictates the amino acid sequence in proteins, which, in turn, determines the structure and function of proteins and ultimately, the characteristics of an organism. The differences in base composition between DNA and RNA contribute to their functional diversity.
3. The Phosphate Group: Linking Nucleotides and Providing Energy
The phosphate group is a negatively charged functional group, (PO₄³⁻), crucial for several reasons:
-
Connecting Nucleotides: The phosphate group links adjacent nucleotides within a nucleic acid chain. This linkage occurs through a phosphodiester bond, where the phosphate group forms a bridge between the 3' carbon of one sugar and the 5' carbon of the next sugar. This creates the characteristic sugar-phosphate backbone of DNA and RNA.
-
Energy Transfer: Nucleotides, especially adenosine triphosphate (ATP), play a central role in energy transfer within cells. ATP is a nucleotide with three phosphate groups. The hydrolysis of the high-energy phosphate bonds in ATP releases energy that fuels a wide array of cellular processes, including muscle contraction, protein synthesis, and active transport. This energy currency of the cell underscores the significance of the phosphate group beyond simply structural integrity.
-
Acidic Nature: The phosphate group contributes to the overall acidic nature of nucleic acids. This acidity influences the molecules' interactions with other cellular components and plays a role in maintaining cellular pH.
The Interplay of the Three Components: Creating Functional Nucleic Acids
The three components of a nucleotide – the pentose sugar, nitrogenous base, and phosphate group – work in concert to create the functional molecules of DNA and RNA. The sugar-phosphate backbone provides structural support, while the sequence of nitrogenous bases encodes genetic information. The phosphate group's role in connecting nucleotides and in energy transfer highlights its crucial contributions beyond simply structural scaffolding.
The specific combination of these three components determines the type of nucleotide and its role within the cell. The precise sequence of nucleotides along the DNA or RNA strand then dictates the genetic information, determining the characteristics and functions of an organism.
Nucleotide Analogs: Expanding the Understanding and Applications
Nucleotide analogs are synthetic molecules that mimic the structure of natural nucleotides. These analogs have become incredibly valuable tools in various applications:
-
Antiviral Drugs: Some nucleotide analogs are employed as antiviral agents. They can interfere with viral replication by incorporating into the viral genome, causing errors in DNA or RNA synthesis. This approach disrupts the viral lifecycle, preventing infection or slowing its progression.
-
Anticancer Drugs: Certain nucleotide analogs are used in cancer therapy. They work by inhibiting DNA synthesis in rapidly dividing cancer cells. This selective targeting can reduce the growth and spread of cancerous tissue.
-
Research Tools: Nucleotide analogs serve as valuable tools in molecular biology research. They can be used to label nucleic acids for detection, to study DNA and RNA synthesis, and to investigate various aspects of gene expression and regulation.
Conclusion: The Fundamental Role of Nucleotides in Life
In essence, nucleotides are the fundamental building blocks of life. Their three components—the pentose sugar, nitrogenous base, and phosphate group—work in concert to form the nucleic acids DNA and RNA, the molecules that store and transmit genetic information, and the machinery that translates it into functional proteins. The precise structure of each component, and their interplay, determine the unique properties of DNA and RNA. A deep understanding of nucleotides and their components remains essential for ongoing breakthroughs in medicine, biotechnology, and our fundamental comprehension of life itself. The ongoing research into nucleotide analogs further expands our toolkit for combating diseases and advancing our understanding of molecular processes within the cell. From the stability of the DNA double helix to the energetic power of ATP, the three components of nucleotides are intricately linked to the remarkable complexity and beauty of life.
Latest Posts
Latest Posts
-
Words That End With A S
May 09, 2025
-
25 Is 50 Of What Number
May 09, 2025
-
What Is 9 Percent In Decimal Form
May 09, 2025
-
5 Letter Word Containing S And I
May 09, 2025
-
Difference Between Experimental Probability And Theoretical Probability
May 09, 2025
Related Post
Thank you for visiting our website which covers about List The Three Parts Of A Nucleotide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.