Lewis Acid And Base Vs Bronsted
Juapaving
Apr 08, 2025 · 6 min read
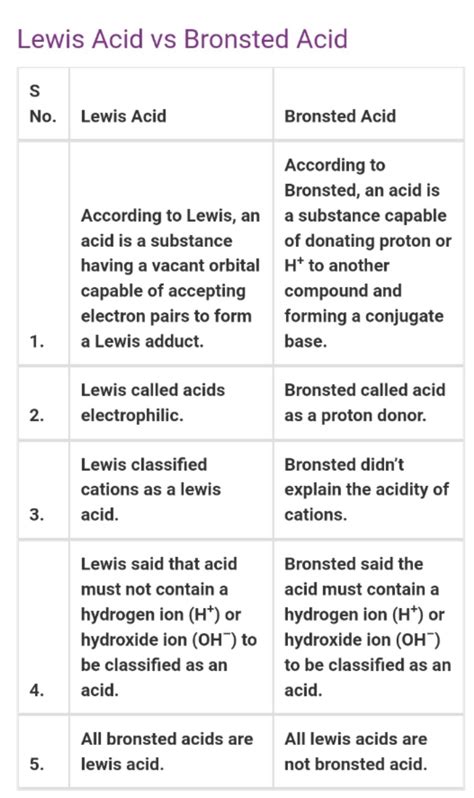
Table of Contents
Lewis Acid and Base vs. Brønsted-Lowry: A Comprehensive Comparison
The concepts of acids and bases are fundamental in chemistry, underpinning countless reactions and processes. While seemingly simple at first glance, the definitions of acids and bases have evolved over time, leading to multiple theories offering different perspectives on this crucial topic. This article delves deep into the comparison between two prominent theories: the Brønsted-Lowry acid-base theory and the Lewis acid-base theory. We will explore their similarities, differences, and the advantages and limitations of each approach, illustrating their applications with real-world examples.
Understanding Brønsted-Lowry Acid-Base Theory
The Brønsted-Lowry theory, proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923, broadened the scope of acid-base chemistry beyond the limitations of the Arrhenius definition. The Arrhenius definition, while useful, restricted acids to substances that produce hydrogen ions (H⁺) in aqueous solutions and bases to those that produce hydroxide ions (OH⁻). The Brønsted-Lowry theory elegantly circumvents this limitation.
Core Principles of Brønsted-Lowry Theory:
- Acid: A Brønsted-Lowry acid is defined as a proton (H⁺) donor. It is a molecule or ion that readily donates a proton to another species.
- Base: A Brønsted-Lowry base is defined as a proton (H⁺) acceptor. It is a molecule or ion that readily accepts a proton from another species.
- Conjugate Acid-Base Pairs: A crucial aspect of the Brønsted-Lowry theory is the concept of conjugate acid-base pairs. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. These pairs are related by the difference of a single proton.
Example:
Consider the reaction between hydrochloric acid (HCl) and water (H₂O):
HCl(aq) + H₂O(l) ⇌ H₃O⁺(aq) + Cl⁻(aq)
In this reaction:
- HCl acts as a Brønsted-Lowry acid, donating a proton to water.
- H₂O acts as a Brønsted-Lowry base, accepting a proton from HCl.
- H₃O⁺ (hydronium ion) is the conjugate acid of H₂O.
- Cl⁻ (chloride ion) is the conjugate base of HCl.
Advantages of Brønsted-Lowry Theory:
- Expands the definition of acids and bases: It doesn't restrict acids and bases to aqueous solutions, encompassing reactions in other solvents or even the gas phase.
- Introduces conjugate acid-base pairs: This concept is crucial for understanding acid-base equilibria and predicting reaction outcomes.
- Simplifies many acid-base reactions: It provides a clear and concise framework for analyzing a wide range of acid-base reactions.
Understanding Lewis Acid-Base Theory
Gilbert N. Lewis proposed an even broader definition of acids and bases in 1923, focusing on the concept of electron pairs rather than proton transfer. This theory encompasses a wider range of reactions than the Brønsted-Lowry theory.
Core Principles of Lewis Acid-Base Theory:
- Lewis Acid: A Lewis acid is defined as an electron-pair acceptor. It is a species that can accept a pair of electrons to form a coordinate covalent bond. Lewis acids often have an incomplete octet or a vacant orbital capable of accepting electron pairs.
- Lewis Base: A Lewis base is defined as an electron-pair donor. It is a species with a lone pair of electrons that can be donated to form a coordinate covalent bond.
Example:
Consider the reaction between boron trifluoride (BF₃) and ammonia (NH₃):
BF₃ + NH₃ → F₃B-NH₃
In this reaction:
- BF₃ acts as a Lewis acid, accepting a lone pair of electrons from ammonia. Boron in BF₃ has only six valence electrons, making it electron-deficient.
- NH₃ acts as a Lewis base, donating a lone pair of electrons to boron.
Advantages of Lewis Acid-Base Theory:
- Most encompassing definition: It includes all Brønsted-Lowry acid-base reactions but also extends to reactions that don't involve proton transfer.
- Explains reactions involving non-protic species: It can explain reactions involving species that lack acidic protons, such as metal cations or certain organic molecules.
- Useful in various fields: It finds broad application in organic chemistry, inorganic chemistry, and biochemistry.
Limitations of Lewis Acid-Base Theory:
- Complexity: The broader definition can make the classification of Lewis acids and bases more complex in some instances compared to Brønsted-Lowry theory. Determining the electron-pair acceptor or donor can sometimes be subjective.
Comparing Brønsted-Lowry and Lewis Acid-Base Theories
| Feature | Brønsted-Lowry | Lewis |
|---|---|---|
| Definition of Acid | Proton donor | Electron-pair acceptor |
| Definition of Base | Proton acceptor | Electron-pair donor |
| Scope | Limited to reactions involving proton transfer | Includes all Brønsted-Lowry reactions plus many others |
| Examples of Acids | HCl, H₂SO₄, CH₃COOH | BF₃, AlCl₃, Fe³⁺ |
| Examples of Bases | NaOH, NH₃, H₂O | NH₃, H₂O, Cl⁻ |
| Conjugate Pairs | Yes | No (although adducts can be considered analogous) |
| Emphasis | Proton transfer | Electron pair sharing |
Applications and Real-World Examples
Both theories are vital tools in chemistry, with applications across various fields. Here are some examples illustrating their practical use:
Brønsted-Lowry Theory Applications:
- Acid-base titrations: These are fundamental techniques in analytical chemistry, relying on the neutralization reaction between an acid and a base, governed by Brønsted-Lowry principles.
- Buffer solutions: Buffers are solutions that resist changes in pH, playing a vital role in biological systems and chemical processes. Their behavior is explained using the Brønsted-Lowry framework.
- Understanding pH and pKa: The pH scale and the pKa values (acid dissociation constants) are directly related to Brønsted-Lowry acid-base equilibria.
Lewis Acid-Base Theory Applications:
- Catalysis: Many chemical reactions are catalyzed by Lewis acids, such as in Friedel-Crafts alkylation and acylation reactions in organic chemistry. The ability of Lewis acids to accept electron pairs facilitates bond formation and reaction pathways.
- Coordination chemistry: Coordination complexes, crucial in many fields (medicine, materials science), involve Lewis acid-base interactions between metal ions (Lewis acids) and ligands (Lewis bases).
- Biological systems: Many biochemical processes involve Lewis acid-base interactions. Enzyme-substrate interactions, for example, often rely on the Lewis acid-base properties of the molecules involved.
Conclusion
Both the Brønsted-Lowry and Lewis acid-base theories offer valuable perspectives on acid-base chemistry. While Brønsted-Lowry theory is a more straightforward approach, focusing on proton transfer, the Lewis theory provides a broader, more inclusive definition encompassing a wider range of reactions. The choice of which theory to apply depends on the specific context and the nature of the chemical reaction being considered. Understanding both theories is essential for a comprehensive grasp of acid-base chemistry and its applications in various scientific and technological domains. The Lewis theory, with its emphasis on electron pair donation and acceptance, offers a powerful lens for analyzing a broader spectrum of chemical interactions, expanding our understanding of chemical bonding and reactivity. Both theories, however, are valuable tools in the chemist's arsenal and provide complementary ways of understanding the fundamental interactions that drive countless chemical processes.
Latest Posts
Latest Posts
-
1 Meter Equals How Many Millimeters
Apr 08, 2025
-
What Is The Lcm Of 6 12 15
Apr 08, 2025
-
Convert 0 24 To A Fraction In Simplest Terms
Apr 08, 2025
-
How Many Lone Pairs Does Oxygen Have
Apr 08, 2025
-
How Many Hours Is 1 Week
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Lewis Acid And Base Vs Bronsted . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.