Law Of Conservation Of Mass In A Sentence
Juapaving
Mar 10, 2025 · 7 min read
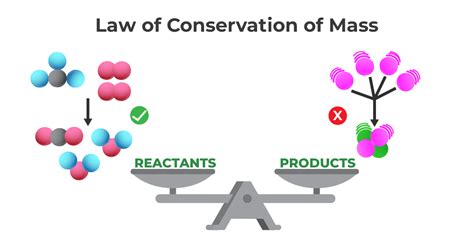
Table of Contents
The Law of Conservation of Mass: A Comprehensive Exploration
The law of conservation of mass, in a single sentence, states that matter cannot be created or destroyed in a chemical reaction, only transformed from one form to another. This seemingly simple statement underpins a vast amount of scientific understanding, impacting fields from chemistry and physics to environmental science and engineering. This article will delve deep into the nuances of this fundamental law, exploring its history, applications, limitations, and ongoing relevance in modern science.
The Genesis of the Law: Lavoisier's Contribution
While the concept of conservation was hinted at by earlier alchemists and scientists, it was Antoine-Laurent Lavoisier, a prominent 18th-century French chemist, who is credited with establishing the law of conservation of mass definitively. Through meticulous experimentation, particularly his work with combustion reactions, Lavoisier demonstrated that the total mass of reactants in a chemical reaction always equals the total mass of the products. He meticulously measured the mass of substances before and after reactions, showing a consistent equivalence, dispelling earlier notions of phlogiston – a hypothetical substance believed to be lost during combustion. Lavoisier's rigorous approach to quantitative analysis laid the foundation for modern chemistry and solidified the law of conservation of mass as a cornerstone principle.
Beyond Combustion: Expanding the Scope
Lavoisier's work focused primarily on combustion, but the law's application extends far beyond this specific reaction type. It holds true for a vast array of chemical processes, including synthesis, decomposition, single displacement, and double displacement reactions. In a synthesis reaction, for example, where multiple reactants combine to form a single product, the total mass remains constant. Similarly, in decomposition reactions, where a compound breaks down into simpler substances, the total mass before and after the reaction remains unchanged. The law is equally applicable to complex multi-step reactions, provided all reactants and products are accounted for.
Understanding the Transformation of Matter
The crucial element of the law is not simply the conservation of mass, but the transformation of matter. Chemical reactions involve the rearrangement of atoms and molecules, not the creation or destruction of matter itself. Consider the simple reaction of hydrogen and oxygen to form water: 2H₂ + O₂ → 2H₂O. Two molecules of hydrogen gas (H₂) react with one molecule of oxygen gas (O₂) to produce two molecules of water (H₂O). While the arrangement of atoms changes drastically, the total number of hydrogen and oxygen atoms remains constant throughout the reaction. The mass before and after the reaction, therefore, remains the same. This principle applies across all chemical transformations.
The Role of Atoms and Molecules
The law of conservation of mass finds its most fundamental explanation at the atomic level. Atoms, the basic building blocks of matter, are neither created nor destroyed in chemical reactions. They simply rearrange themselves to form new molecules. This atomic perspective provides a robust underpinning to the law, solidifying its position as a fundamental principle of chemistry. The rearrangement of these atoms during chemical reactions leads to changes in the physical and chemical properties of the substances involved, but the total mass remains constant due to the conservation of atoms.
Practical Applications and Real-World Examples
The law of conservation of mass has far-reaching implications and practical applications across various fields.
Chemistry and Industrial Processes:
Chemical engineers rely heavily on the law to design and optimize industrial processes. Accurate mass balances are essential for efficient production and waste management. Knowing that mass is conserved allows for precise calculations of reactant quantities required to yield a specific amount of product, minimizing waste and maximizing efficiency. This principle is critical in industries ranging from pharmaceuticals and petrochemicals to food processing and materials science.
Environmental Science:
In environmental science, the law is crucial for understanding pollution and environmental remediation. Tracking the movement and transformation of pollutants relies on the principle of mass conservation. For example, understanding how much of a particular pollutant is released into the environment and how it transforms into other compounds requires applying the law to monitor its overall quantity. This knowledge is essential for developing effective environmental policies and remediation strategies.
Forensic Science:
Forensic science also utilizes the principle of mass conservation. In crime scene investigations, determining the mass of substances before and after a reaction can help reconstruct events and provide crucial evidence. For example, analyzing the mass of residues left at a crime scene, understanding how it relates to its origin, and determining whether there were any transformations or reactions, all utilize the principle of mass conservation in crime scene investigation.
Limitations and Considerations
While the law of conservation of mass is a powerful principle, it does have some limitations:
Nuclear Reactions:
The law does not apply to nuclear reactions. In nuclear reactions, a small amount of mass is converted into energy, as described by Einstein's famous equation, E=mc². This mass-energy conversion means that mass is not strictly conserved in nuclear processes. Nuclear fission and fusion reactions are prime examples where a measurable difference in mass is observed, demonstrating that a small amount of mass is indeed converted to energy during such processes.
Open Systems:
The law only strictly applies to closed systems. In open systems, where matter can enter or leave, the total mass within the system may change over time. For instance, if you are boiling water in an open container, some of the water will evaporate. In open systems, mass is not strictly conserved, as substances can be added or removed during the reaction, thus violating the condition of the closed system required by the law of conservation of mass. This implies that any system in which the total amount of mass increases or decreases because of substances leaving or entering the system will violate the law.
The Law's Enduring Relevance in Modern Science
Despite its limitations, the law of conservation of mass remains a fundamental cornerstone of chemistry and other related sciences. It provides a crucial framework for understanding chemical reactions and their applications. Even in the context of nuclear reactions, where mass-energy equivalence comes into play, the overall conservation principle still holds true, albeit in a more complex form. The modified concept is that mass-energy is conserved in all processes.
The law continues to be essential in modern scientific research and technological advancements across various fields. From the development of new materials to the design of more efficient industrial processes, the understanding of matter conservation is indispensable. Furthermore, its simplicity and wide applicability make it a key concept for teaching foundational scientific principles at various educational levels.
Continued Refinements and Interpretations
While the fundamental principle of mass conservation remains unchanged, its interpretation and application have been refined over time. The incorporation of Einstein's theory of relativity provided a more complete understanding of the relationship between mass and energy, extending the law's scope to include nuclear phenomena. Modern scientific instruments allow for even more precise measurements of mass changes during chemical reactions, further confirming the validity of the law within its defined boundaries.
Conclusion: The Power of a Simple Principle
The law of conservation of mass, stated concisely as "matter cannot be created or destroyed in a chemical reaction, only transformed from one form to another," is a deceptively simple yet profoundly powerful principle. Its historical development, diverse applications, and enduring relevance in modern science underscore its critical role in our understanding of the physical world. While its limitations in the context of nuclear reactions and open systems are acknowledged, its fundamental importance in chemistry, environmental science, and other fields remains undisputed. The law serves as a testament to the power of scientific observation, meticulous experimentation, and the ongoing refinement of scientific understanding. It provides a foundational framework upon which many other scientific principles are built, cementing its place as a cornerstone of modern science.
Latest Posts
Latest Posts
-
How Many Degrees Are In A Half Circle
May 09, 2025
-
What Force Keeps The Planets In Orbit Around The Sun
May 09, 2025
-
What Are The Products Of This Chemical Reaction
May 09, 2025
-
What Are Some Examples Of A Screw
May 09, 2025
-
What Planet Is Known As The Morning Star
May 09, 2025
Related Post
Thank you for visiting our website which covers about Law Of Conservation Of Mass In A Sentence . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.