Isotopes Of The Same Element Have Different
Juapaving
Apr 06, 2025 · 7 min read
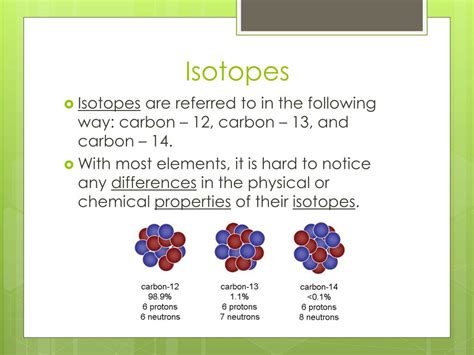
Table of Contents
Isotopes of the Same Element Have Different: A Deep Dive into Nuclear Variations
Isotopes are atoms of the same element that share the same number of protons but differ in the number of neutrons. This seemingly subtle difference leads to a fascinating array of variations in their properties, impacting everything from their stability to their applications in various fields. Understanding these differences is crucial in numerous scientific disciplines, from nuclear physics and chemistry to medicine and geology.
The Fundamentals: Protons, Neutrons, and Atomic Mass
To grasp the differences between isotopes, we must first understand the basic structure of an atom. An atom consists of a nucleus containing protons and neutrons, surrounded by orbiting electrons. The number of protons defines the atomic number, which determines the element's identity. For instance, all atoms with one proton are hydrogen, those with six are carbon, and those with 92 are uranium.
Neutrons, on the other hand, don't affect the element's identity. They contribute to the atom's mass number, which is the sum of protons and neutrons. Isotopes are variations of the same element that possess the same number of protons but differ in their neutron count. This difference in neutron number directly impacts several key properties.
Key Differences Between Isotopes:
-
Atomic Mass: This is perhaps the most obvious difference. Since isotopes differ in their neutron number, their atomic mass also varies. This is typically expressed in atomic mass units (amu), with one amu approximately equal to the mass of a proton or neutron. For example, carbon-12 (¹²C) has 6 protons and 6 neutrons, while carbon-14 (¹⁴C) has 6 protons and 8 neutrons. ¹⁴C has a higher atomic mass than ¹²C.
-
Nuclear Stability: The neutron-to-proton ratio plays a crucial role in determining the stability of an atom's nucleus. Many isotopes are stable, meaning their nuclei do not spontaneously decay. However, many isotopes are unstable or radioactive, meaning their nuclei spontaneously decay, emitting particles and energy in the process. This decay transforms the unstable isotope into a different element or a more stable isotope. The rate of decay is characterized by the isotope's half-life.
- Radioactive Decay: This process involves the emission of alpha particles (helium nuclei), beta particles (electrons or positrons), or gamma rays (high-energy photons). The type of decay an isotope undergoes is determined by its neutron-to-proton ratio and the energy levels within its nucleus. The energy released during radioactive decay can be harnessed for various applications.
-
Physical Properties: While the chemical properties of isotopes of the same element are virtually identical (due to the same number of electrons), their physical properties can differ slightly. This is primarily due to the difference in mass. For example, isotopes with higher masses might have slightly different melting and boiling points, densities, and diffusion rates. These differences, however, are usually subtle and often negligible in many practical applications.
-
Chemical Properties: Since isotopes of the same element have the same number of electrons, their chemical behavior is essentially the same. This is because chemical reactions involve the interaction of electrons, not the nucleus. This is why isotopes of an element participate in the same chemical reactions and form the same types of compounds. However, subtle differences in reaction rates can sometimes be observed due to the kinetic isotope effect, primarily influenced by the mass difference.
-
Nuclear Reactions: Isotopes play a crucial role in nuclear reactions, including nuclear fission and fusion. Specific isotopes are chosen for their suitability in these processes due to their unique nuclear properties and stability. For example, Uranium-235 (²³⁵U) is commonly used in nuclear fission reactors due to its high fissile nature, while deuterium (²H) and tritium (³H) are used in nuclear fusion experiments.
Applications of Isotopic Differences:
The unique properties of isotopes make them invaluable tools in various scientific and technological applications:
1. Radioactive Dating:
Radioactive isotopes with known half-lives are used to determine the age of materials. This technique, known as radiometric dating, is crucial in archaeology, geology, and paleontology. For example, carbon-14 dating is commonly used to determine the age of organic materials up to around 50,000 years old. Other isotopes, such as uranium-238 and potassium-40, are used to date much older geological formations.
2. Medical Applications:
Radioactive isotopes find extensive use in medical imaging and treatment. Radioactive tracers, which are incorporated into molecules, can be used to track the movement and distribution of these molecules within the body. This technique is employed in various diagnostic procedures, such as PET (positron emission tomography) scans, which utilize isotopes like fluorodeoxyglucose (FDG) labeled with fluorine-18. Radioactive isotopes are also used in radiation therapy to destroy cancerous cells. Iodine-131, for example, is commonly used to treat thyroid cancer.
3. Industrial Applications:
Isotopes are used in various industrial processes, including gauging the thickness of materials, tracing the flow of fluids in pipelines, and analyzing the composition of materials. The use of radioactive isotopes in these applications ensures safety and efficiency.
4. Research Applications:
Isotopes serve as indispensable tools in scientific research across various fields. They are used to study chemical reactions, track the movement of molecules, and understand biological processes at the molecular level. Stable isotopes, particularly those of hydrogen, carbon, nitrogen, and oxygen, are routinely used in stable isotope analysis. This method helps to understand various biological and environmental processes like migration patterns, dietary habits, and climate change.
Specific Examples of Isotopic Differences:
Let's examine some specific examples to illustrate the differences between isotopes more concretely:
1. Hydrogen Isotopes:
- Protium (¹H): The most common isotope of hydrogen, containing one proton and no neutrons.
- Deuterium (²H or D): Contains one proton and one neutron. It is a stable isotope and used in various applications including nuclear fusion research and as a tracer in biological studies. Deuterium oxide (D₂O), also known as heavy water, has slightly different properties compared to ordinary water.
- Tritium (³H or T): Contains one proton and two neutrons. It is radioactive and decays through beta decay with a half-life of approximately 12.3 years. It's used in some nuclear fusion experiments and as a radioactive tracer in various scientific studies.
The difference in mass between protium and deuterium leads to noticeable differences in their physical properties. For example, deuterium oxide has a higher boiling point and density than ordinary water (H₂O).
2. Carbon Isotopes:
- Carbon-12 (¹²C): The most abundant stable isotope of carbon.
- Carbon-13 (¹³C): A stable isotope used in various applications including nuclear magnetic resonance (NMR) spectroscopy and isotopic labeling in biological research.
- Carbon-14 (¹⁴C): A radioactive isotope with a half-life of approximately 5,730 years. Its decay is used in radiocarbon dating to determine the age of organic materials.
The difference in stability between ¹²C and ¹⁴C is significant. ¹²C is a stable isotope, while ¹⁴C undergoes beta decay, transforming into nitrogen-14.
3. Uranium Isotopes:
- Uranium-235 (²³⁵U): A fissile isotope used as fuel in nuclear reactors and nuclear weapons. Its nucleus readily undergoes fission, releasing a large amount of energy.
- Uranium-238 (²³⁸U): The most abundant isotope of uranium. While not fissile, it is fertile, meaning it can be converted into plutonium-239, which is fissile, in a nuclear reactor.
The difference in fissile nature between ²³⁵U and ²³⁸U makes them crucial in nuclear technology.
Conclusion:
Isotopes of the same element, while sharing the same number of protons and hence the same chemical identity, exhibit significant differences in their properties due to variations in neutron numbers. These differences impact their stability, mass, physical properties, and importantly, their applications in various scientific, medical, and industrial fields. From radioactive dating to nuclear energy production and medical diagnostics, isotopes provide invaluable tools that continue to shape our understanding of the world and advance technological innovation. Understanding the fundamental differences between isotopes is therefore crucial for anyone interested in chemistry, physics, biology, geology, or any field utilizing isotopic techniques.
Latest Posts
Latest Posts
-
Do Birds Have Four Chambered Hearts
Apr 09, 2025
-
A Particle Moves Along The X Axis
Apr 09, 2025
-
Bromine Has How Many Valence Electrons
Apr 09, 2025
-
Fluid Pressure Against A Wall Or Cell Membranes Is Called
Apr 09, 2025
-
What Is The Largest Cell Called
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Isotopes Of The Same Element Have Different . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.