Is Water Boiling Endothermic Or Exothermic
Juapaving
Mar 23, 2025 · 5 min read
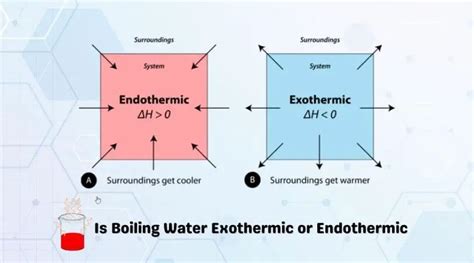
Table of Contents
Is Boiling Water Endothermic or Exothermic? Understanding Heat Transfer in Phase Changes
The question of whether boiling water is an endothermic or exothermic process is a fundamental concept in chemistry and thermodynamics. Understanding this seemingly simple phenomenon unlocks a deeper appreciation of heat transfer and phase changes. This article will delve into the intricacies of this process, explaining the underlying principles, clarifying common misconceptions, and providing practical examples.
Understanding Endothermic and Exothermic Reactions
Before we address the boiling water question, let's define the key terms:
-
Endothermic reactions: These are processes that absorb heat from their surroundings. The system's temperature decreases as it absorbs energy. Think of it like a sponge soaking up water – the sponge (the system) gains energy, and the surrounding area loses some.
-
Exothermic reactions: These are processes that release heat to their surroundings. The system's temperature increases as it releases energy. Imagine a fire – it releases heat (energy) into the environment.
The Phase Change of Water: From Liquid to Gas
Water, in its liquid state, exists as molecules held together by relatively weak intermolecular forces (hydrogen bonds). When we heat water, we're essentially adding energy to these molecules. This added energy increases the kinetic energy of the water molecules, causing them to vibrate and move more rapidly.
As the temperature increases, the kinetic energy eventually overcomes the intermolecular forces holding the water molecules together in the liquid state. This is the point where the water begins to boil. The boiling point of water at standard atmospheric pressure is 100°C (212°F).
During boiling, the added heat energy is used to break these intermolecular forces, allowing the water molecules to escape the liquid phase and transition into the gaseous phase (steam). This transition from liquid to gas is called vaporization.
Why Boiling Water is an Endothermic Process
The key takeaway here is that energy is absorbed during the boiling process. The heat energy isn't increasing the temperature of the water (after it reaches boiling point), but rather is being used to overcome the intermolecular forces and change the phase of the water from liquid to gas. Therefore, boiling water is an endothermic process.
Visualizing the Energy Absorption
Imagine the water molecules as tiny balls connected by springs (representing the intermolecular forces). As you add heat (energy), you're essentially stretching and eventually breaking those springs. This requires energy input – the stretching and breaking of the "springs" (hydrogen bonds) requires energy to be absorbed from the surrounding environment. Once the bonds are broken, the molecules escape as gas, carrying the absorbed energy with them.
The Role of Latent Heat of Vaporization
The amount of energy required to change one gram of a substance from a liquid to a gas at its boiling point is called the latent heat of vaporization. For water, this value is relatively high (approximately 2260 J/g), which means it takes a significant amount of energy to boil water. This high latent heat is directly related to the strength of the hydrogen bonds in water.
This latent heat explains why boiling takes time; it's not just a matter of increasing the temperature; it involves absorbing a substantial amount of energy to overcome the intermolecular forces.
Misconceptions about Boiling Water
Several common misconceptions surround the endothermic nature of boiling water. Let's address some of these:
-
Misconception 1: The temperature keeps rising indefinitely. Once water reaches its boiling point, the added heat energy doesn't increase the temperature further; instead, it's used for vaporization. The temperature remains constant at 100°C (at standard atmospheric pressure) until all the water has boiled away.
-
Misconception 2: Boiling is only about temperature. Boiling is a phase transition, not simply a temperature increase. It requires a significant amount of energy to break the intermolecular forces, even though the temperature remains constant.
-
Misconception 3: Boiling is exothermic because it feels hot. The heat you feel from boiling water is the energy already present in the steam, not energy being released during the boiling process. The steam is already carrying the energy absorbed during vaporization.
Practical Applications and Real-World Examples
The endothermic nature of boiling has numerous practical applications:
-
Cooking: Boiling water is crucial for cooking many foods, taking advantage of the energy transfer during the phase change.
-
Cooling systems: Evaporation is an endothermic process. This principle is utilized in cooling systems such as evaporative coolers (swamp coolers) and sweating. As water evaporates from the skin, it absorbs heat, leading to a cooling effect.
-
Power generation: Steam turbines in power plants utilize the energy released when steam condenses back into water (an exothermic process), driving the turbines to generate electricity. The initial steam generation, however, is endothermic.
-
Steam distillation: This technique uses steam to separate volatile compounds from non-volatile compounds, leveraging the energy absorption during steam generation.
-
Climate regulation: Evaporation of water from oceans and lakes plays a vital role in regulating the Earth's climate. The process absorbs a massive amount of energy, influencing temperature patterns.
Further Exploration: Factors Affecting Boiling Point
While 100°C is the boiling point of water at standard atmospheric pressure, several factors can influence this:
-
Pressure: Lower atmospheric pressure lowers the boiling point of water. At higher altitudes, where the atmospheric pressure is lower, water boils at a lower temperature. This is why it takes longer to cook food at high altitudes.
-
Impurities: Dissolved substances in water can slightly alter its boiling point. Generally, adding solutes increases the boiling point (boiling point elevation).
-
Container material: The material of the container can influence heat transfer, slightly affecting the boiling time but not the inherent endothermic nature of the process.
Conclusion: Boiling – An Endothermic Phase Change
In conclusion, the process of boiling water is unequivocally endothermic. It requires the absorption of heat energy to overcome the intermolecular forces holding the water molecules together in the liquid state, allowing them to transition into the gaseous phase. Understanding this fundamental concept is crucial for comprehending various scientific phenomena and their practical applications in everyday life, from cooking to climate regulation to industrial processes. The high latent heat of vaporization of water underscores the significant energy involved in this phase transition and highlights its importance in many natural and technological systems. By appreciating the nuances of endothermic and exothermic processes, we can gain a more comprehensive understanding of the world around us.
Latest Posts
Latest Posts
-
How Many Red Cards Are There In A Deck
Mar 24, 2025
-
Which Of These Is Considered A Computers Brain
Mar 24, 2025
-
What Is The Charge Of Sn
Mar 24, 2025
-
Is 23 A Prime Or Composite Number
Mar 24, 2025
-
Stomata Appear In Which Group Of Plants
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Is Water Boiling Endothermic Or Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
