Is Water An Element Compound Or Mixture
Juapaving
Mar 06, 2025 · 6 min read
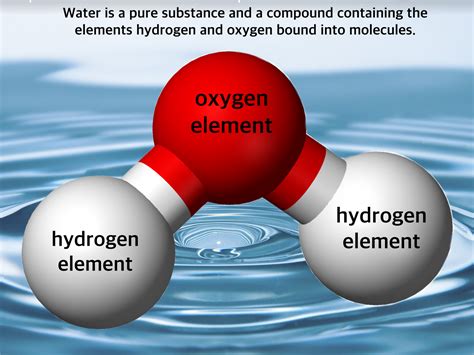
Table of Contents
Is Water an Element, Compound, or Mixture? A Deep Dive into H₂O
The question, "Is water an element, compound, or mixture?" might seem deceptively simple. After all, water is something we encounter daily. However, understanding its true nature requires exploring the fundamental concepts of chemistry. This article will delve into the fascinating world of water's composition, exploring its properties and definitively answering the central question. We'll also touch upon the broader implications of understanding the difference between elements, compounds, and mixtures.
Understanding the Basics: Elements, Compounds, and Mixtures
Before we classify water, let's clarify the definitions of the three categories:
Elements:
Elements are fundamental substances that cannot be broken down into simpler substances by chemical means. They are made up of only one type of atom. Think of the periodic table – each square represents a unique element, such as hydrogen (H), oxygen (O), iron (Fe), gold (Au), etc. These atoms are the building blocks of all matter.
Compounds:
Compounds are formed when two or more different elements chemically combine in a fixed ratio. This chemical combination involves the sharing or transfer of electrons between atoms, creating strong chemical bonds. Unlike mixtures, the elements in a compound lose their individual properties and form a new substance with unique characteristics. The composition of a compound is always consistent. For example, water (H₂O) is always two hydrogen atoms combined with one oxygen atom. You can't change that ratio and still have water.
Mixtures:
Mixtures are combinations of two or more substances that are physically mixed but not chemically combined. Unlike compounds, the components of a mixture retain their individual properties. The composition of a mixture is variable; you can have different ratios of the components. For example, saltwater is a mixture of salt (NaCl) and water (H₂O). The salt and water retain their individual properties, and the ratio of salt to water can vary. Mixtures can be homogeneous (uniform throughout, like saltwater) or heterogeneous (non-uniform, like sand and water).
The Case of Water: H₂O
Now, let's analyze water (H₂O) using the above definitions. Water is composed of two elements: hydrogen (H) and oxygen (O). These elements are chemically bonded together in a fixed ratio: two hydrogen atoms for every one oxygen atom. This bond is a covalent bond, where the hydrogen and oxygen atoms share electrons to achieve a stable electron configuration. This chemical bonding results in the formation of a new substance – water – with distinct properties different from hydrogen and oxygen. Hydrogen is a highly flammable gas, and oxygen is a vital gas for respiration. Water, however, is a liquid at room temperature and essential for life. This clearly demonstrates that water is not a mere physical mixture of hydrogen and oxygen; it is a chemically bonded compound.
Why Water is a Compound, Not a Mixture
Several key characteristics solidify water's classification as a compound:
- Fixed Ratio: The ratio of hydrogen to oxygen in water is always 2:1. You cannot have water with a different ratio of these elements. This fixed ratio is a defining characteristic of compounds.
- New Properties: Water exhibits properties distinctly different from hydrogen and oxygen. It's a liquid at room temperature, a universal solvent, and essential for life. These properties are not simply an average of the properties of hydrogen and oxygen.
- Chemical Bonding: Hydrogen and oxygen atoms are held together by strong covalent bonds. To separate them, a chemical reaction (such as electrolysis) is required. This is not the case with mixtures, which can be separated by physical means (like filtration or distillation).
- Uniform Composition: Pure water is a homogeneous substance, meaning it has a uniform composition throughout. This uniformity is another indicator of a compound.
The Importance of Understanding the Distinction
Differentiating between elements, compounds, and mixtures is crucial in various scientific fields:
- Chemistry: Understanding the composition of substances helps us predict their reactions and properties, enabling the development of new materials and technologies.
- Biology: The chemical nature of water is fundamental to biological processes. Its unique properties facilitate metabolic reactions and maintain cellular structures.
- Environmental Science: Knowledge of chemical compounds and mixtures is essential for understanding pollution, water treatment, and the overall health of our planet.
- Material Science: The properties of materials are closely related to their composition, whether it's a pure element, a compound, or a mixture.
Water's Unique Properties: A Consequence of its Compound Nature
Water's unique properties, essential for life as we know it, are directly linked to its molecular structure as a compound:
- High Specific Heat Capacity: Water can absorb significant amounts of heat without a large temperature change. This property moderates temperature fluctuations in aquatic environments and within living organisms.
- High Heat of Vaporization: A large amount of heat is required to change water from liquid to gas (evaporation). This property contributes to evaporative cooling, crucial for temperature regulation in many systems.
- Density Anomaly: Ice is less dense than liquid water, causing it to float. This property insulates aquatic life during freezing temperatures, preventing bodies of water from freezing solid.
- Excellent Solvent: Water's polar nature makes it an excellent solvent, dissolving many ionic and polar substances. This property is essential for transporting nutrients and waste products in biological systems.
- Cohesion and Adhesion: Water molecules stick to each other (cohesion) and to other surfaces (adhesion). These properties are crucial for capillary action in plants and the surface tension of water.
Beyond the Basics: Isotopes and Water
While the chemical formula for water is always H₂O, variations exist due to isotopes. Isotopes are atoms of the same element with differing numbers of neutrons. Hydrogen has three isotopes: protium (¹H), deuterium (²H), and tritium (³H). Oxygen also has several isotopes. This means that "water" can actually encompass various isotopic combinations, such as H₂¹⁶O, H₂¹⁸O, D₂O (heavy water), and others. These variations have subtle but important implications in various scientific applications.
Conclusion: Water - The Essential Compound
In conclusion, water is unequivocally a compound, not an element or a mixture. Its unique properties, crucial for life and numerous applications, arise directly from its chemical composition: two hydrogen atoms covalently bonded to one oxygen atom. Understanding this fundamental classification is essential for advancing our knowledge in various scientific disciplines and for appreciating the critical role water plays in our world. The seemingly simple question of classifying water opens a window into the complex and fascinating world of chemistry and its profound impact on our lives. Further exploration of water's properties and behavior will continue to unveil its secrets and enhance our understanding of the natural world.
Latest Posts
Latest Posts
-
What Is The Lcm Of 6 And 5
Mar 06, 2025
-
Lowest Common Factor Of 6 And 10
Mar 06, 2025
-
Which Of The Following Are Examples Of Chemical Changes
Mar 06, 2025
-
Is 37 A Prime Or Composite
Mar 06, 2025
-
Is Tearing Physical Or Chemical Change
Mar 06, 2025
Related Post
Thank you for visiting our website which covers about Is Water An Element Compound Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
